The effect of feeling full and reducing appetite even if you eat just a little food… Administered once a week as prescribed by a doctor
Mr. A, who started dieting in July, recently booked a prescription for the obesity treatment drug ‘We Gobi’ at a local hospital. I tried many times to lose weight, but it wasn’t very effective. I tried to control the amount of food I ate and make exercise a part of my daily routine. Instead of taking the elevator, I went up and down the stairs to my 8th floor house, and every time I picked up household waste, I walked down and threw it away. I tried appetite suppressants, but I developed tolerance and they were less effective. Then I recently heard the news that WeGobee would be released in Korea in October. Although the price is a bit burdensome, Mr. A plans to take Wigobi soon.
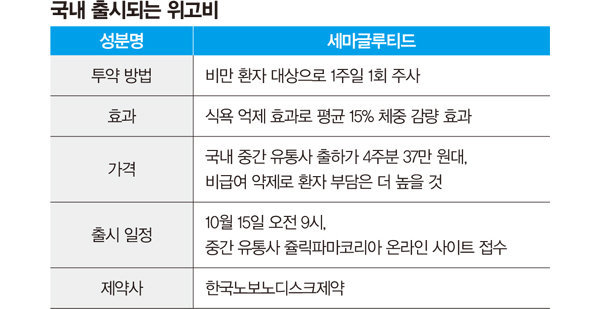
‘WeGobee’ (ingredient name: semaglutide), an obesity treatment selected by Tesla CEO Elon Musk, will be introduced to Korea in mid-October. Zuellig Pharma Korea, which is in charge of intermediate distribution, will begin accepting orders for Wegobi through its online site from 9 a.m. on October 15. It is called a ‘game changer’ in that it is a groundbreaking treatment that can be expected to reduce weight with just one injection a week, and there is a strong expectation that it will change the market. There is concern that sales prices will rise due to high demand but insufficient domestic supply.

15% weight loss when taking medication once a week for 1.5 years
Wigobi has the effect of making you feel full easily and reducing your appetite even if you eat only a small amount of food. This is because semaglutide, the main ingredient in Wigobi, is a drug made by mimicking the hormone glucagon-like peptide (GLP-1). Heo Yang-im, a professor of family medicine at Bundang Cha Medical Center, said, “Originally, GLP-1 is a hormone released from the stomach that promotes insulin secretion and plays a role in slowing down digestion.” He added, “Gastrogobi, which mimics this hormone, is also a GLP-1 drug, so it reduces eating. “I feel like digestion is slowing down and I feel full,” he explained.
Clinical results showed that even with just one injection per week, the average body weight decreased by about 15% after 68 weeks. Even a 120 kg person can gain weight in the low 100 kg range if they receive injections for a year and a half. Compared to the existing obesity treatment drug ‘Saxenda’, it is superior in terms of simplicity and weight loss effect. Saxenda must be administered daily, but Wigovi requires injection once a week. Saxenda shows an average weight loss of 7.5% over 56 weeks of treatment, while Wegobi shows a 14.9% weight loss over 68 weeks of treatment. Kang Jae-heon, a professor of family medicine at Kangbuk Samsung Hospital, said, “Both are drugs from the same company (Novo Nordisk), and many studies have shown that Wigobi has more than twice the weight loss effect than Saxenda.”
Although the weight loss effect is dramatic, the medical community is paying attention to the effect of improving secondary diseases caused by obesity. Professor Heo said, “Among the existing obesity treatments, there were products like ‘sibutramine’ that reduce body weight but have a high risk of developing heart disease, so there were many problems,” adding, “WeGobi is attracting attention as it can not only help with diet, but also prevent cardiovascular disease and reduce complications caused by obesity.” “I’m receiving it,” he said.
Thanks to its noticeable weight loss effect, Wegobi is also known as a drug taken by celebrities in the United States. In 2022, Musk posted a post titled “Fasting and Wegovy” on social networking service Musk, who was in good shape, lost 13.6 kg, and it became known that broadcaster Oprah Winfrey and model/actress Kim Kardashian also went on diets, leading to shortages.
Dosing starts with small dosesDistributors launching WeGobee in Korea will accept orders from hospitals, clinics and pharmacies starting October 15 (see table). Prescriptions for actual patients are expected to begin around late October. There are 5 doses: 0.25 mg, 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg. It is an injection method that looks like a pen and is administered once a week. It is expected that the prescription will be administered in a small amount and gradually increased.
The target of prescription is ‘obese patients’ with a body mass index (BMI: weight divided by the square of height) of 30㎏/㎡ or more. It can also be prescribed for weight loss purposes to patients who are overweight with a BMI of 27 kg/m2 or more to less than 30 kg/m2 and have one or more concomitant diseases (diabetes, high blood pressure, hyperlipidemia, fatty liver, etc.).
The domestic supply price of Wegobee is 370,000 won. It is excluded from insurance coverage and the patient bears the full cost of the drug. If it is prescribed only for the purpose of treating obesity without the purpose of treating other diseases such as cardiovascular disease, it is not expected to be included in actual cost insurance from private insurance companies. Adding distribution costs, medical treatment costs, and prescription costs, the actual cost borne by patients is expected to be in the range of 800,000 to 1 million won per four weeks. A medical industry official said, “I don’t know what the commercial price is, but I was surprised that the supply price seemed to be much cheaper than expected.”
Be aware of side effects when using. The side effects known so far are gastrointestinal symptoms such as nausea, vomiting, abdominal pain, diarrhea, and constipation. The most common side effects are due to changes in bowel movement. Professor Heo explained, “You need to start with a small amount and increase it to see if you adapt.” Professor Kang advised, “No fatal side effects have been found yet, but it is a drug that has only been on the market for about 10 years, so we need to watch it a little more and use it with caution.”
The obesity rate among Korean adults has been increasing recently. According to the Korea Disease Control and Prevention Agency’s National Health and Nutrition Survey, the obesity rate among Korean adults increased from 30.9% in 2014 to 37.2% in 2022. According to the Korean Obesity Society’s ‘2023 Obesity Fact Sheet’, the overall prevalence of obesity in adults has been increasing over the past 10 years. The overall prevalence of obesity in adults was 30.6% in 2013, 35.7% in 2018, and 38.4% in 2022. The prevalence of obesity in men was 37.9% in 2013, 45.4% in 2018, and 49.6% in 2022.
As the obesity rate increases, the number of prescriptions for the obesity treatment drug ‘Saxenda’ is also increasing. Looking at the status of Saxenda prescriptions received by Representative Seo Young-seok of the Democratic Party of Korea from the Health Insurance Review and Assessment Service, the number of prescriptions increased significantly from 97,091 in 2019 to 90,112 in 2021 and 171,223 in 2023. This year, the number of cases counted as of June reached 94,884. Professor Heo said, “Obesity is not just a matter of weight, but also a huge problem of medical costs resulting from cardiovascular disease. To lower these costs, we need to review whether or not obesity treatment drugs for therapeutic purposes are covered by insurance.”
Domestic pharmaceutical companies are also developing obesity treatments one after another.
Domestic pharmaceutical companies are also busy developing obesity treatments. Hanmi Pharmaceutical is developing obesity treatment drugs at a rapid pace. Like Wegobee, we have developed ‘Efpeglenatide’, a GLP-1 type obesity treatment, and it is currently in the phase 3 clinical trials in Korea. An official from Hanmi Pharmaceutical said, “We plan to complete clinical trials at the end of 2026 and commercialize it in 2027.”
Yuhan Corporation is preparing for phase 1 clinical trials in the U.S. for an obesity treatment candidate. The candidate substance is the GLP-1 series, and in previous clinical trials, it showed a weight loss effect of 11.9%, which is higher than Wigobi (6%). In addition, Ildong Pharmaceutical, Daewoong Pharmaceutical, and Daewon Pharmaceutical are known to be developing capsule-type and patch-type obesity medicines that are differentiated from obesity treatments currently on the market.
Experts emphasize that if you want to avoid yo-yoing while losing weight, you should not rely solely on obesity treatments. Professor Kang said, “Even if this drug has a better weight loss effect than existing drugs, all obesity treatments must be combined with diet control and exercise. Long-term effects can be expected only by maintaining a healthy lifestyle and not relying solely on drugs.” He requested.

*If you search and follow ‘Magazine Jin Donga’ and ‘Two Avengers’ on YouTube and portal sites respectively, you can find a variety of investment information such as videos in addition to articles.
《This article Weekly Donga It was published in issue 1460》
Reporter Yoon Chae-won, Publication Bureau [email protected]
-
- great
- 0dog
-
- I’m sad
- 0dog
-
- I’m angry
- 0dog
-
- I recommend it
- dog

