WIGP Pharmaceutical, domestically producing disposable endoscope disinfectant… Item approval in December last year
Recently selected and reviewed distributors… Sales and supply begin in earnest
Voices for strengthening guidelines and supervision for disinfection and management of disposable endoscopes
This year’s National Assembly inspection reveals the current status of disposable endoscope management… Approximately 500 locations are inadequate
WIGP Pharmaceutical and Choyang Medical, joint technology sales for disinfectant and sterilizer
In this year’s National Assembly audit, the reuse of disinfectant solution for endoscopic instruments became a hot topic.
According to the Ministry of Health and Welfare’s manual, the effective concentration of the disinfectant is tested once a day and if it does not meet the standards, it is managed by discarding it. However, instruments that have been inside other people’s bodies are washed with the same disinfectant that has been used dozens of times, and the instruments may end up in the body or someone else’s body. It still feels uncomfortable when I think about it entering my body. Moreover, it is shocking that hundreds of domestic examination institutions have been judged unsuitable in the disinfection inspection of gastrointestinal and colonoscopy equipment. Overseas, Europe has changed its disinfectants from multi-use to disposable, and the United States is also said to be expanding the use of disposable disinfectants. On the other hand, domestic hospitals and clinics only use multi-use disinfectants.
Meanwhile, WIGP Pharmaceutical Co., Ltd., a domestic medical device company, is attracting attention after successfully domestically producing disposable endoscope disinfectant solution last year. In order to use disposable disinfectant, it must be replaced with dedicated equipment, but the cost burden is not large, and since disposable disinfectant is used less than multi-use based on the same number of times, there is no significant difference in the cost of purchasing disinfectant.

WIGP Pharmaceutical begins sales of disposable endoscope disinfectant… Promotion of distributor agreement
According to the industry on the 14th, WIGP Pharmaceutical is pursuing full-scale sales of ‘We Clean PA 15 solution (peracetic acid solution)’, a high-capacity disposable endoscope disinfectant solution that obtained product approval from the Ministry of Food and Drug Safety (MFDS) in December last year. It is known that several pharmaceutical companies are showing interest in distributing the product, and some of them have begun reviewing the details of the specific distribution contract. Asan Pharmaceutical is said to have decided to supply disinfectant products separately from the distributor. Since the disposable endoscope disinfectant solution uses highly concentrated peracetic acid, the performance and durability of the endoscope sterilizer are important. Therefore, domestic hospitals and clinics that have been using multi-use disinfectants must replace their disinfection equipment separately. Weclean PA15 liquid can sterilize endoscope instruments using the disposable self-cleaning sterilizer ‘CYW-S100’ developed by Joyang Medical Industries. In fact, WIGP Pharmaceutical and Choyang Medical Industries are said to be cooperating as a team in the sales field. CYW-S100 is a medical device for disinfecting disposable endoscopes and is the latest equipment developed in accordance with infection control and disinfection standards. This method mixes 80ml of Weaklean PA 15 liquid with 7920ml of water and automatically dries after disinfection. Functionally, the cleaning and disinfection power has been improved by applying a 360-degree rotating multi-channel injection system and a standard bacterial growth micron (0.2㎛) water filter. In addition, the intuitive operation system was adopted to increase convenience of use.

The inconvenient truth ‘Disinfection of endoscope instruments’… This year’s National Assembly inspection management situation is on the chopping block.
In this National Assembly inspection, the reuse of endoscope instrument disinfectants was a ‘hot potato’. The assessment is that an uncomfortable truth was revealed in a field that no one was interested in. According to data submitted by People Power Party lawmaker Baek Jong-heon, a member of the National Assembly Health and Welfare Committee (Welfare Committee), from the National Health Insurance Corporation, gastric and colonoscopy equipment was disinfected at 28,783 examination institutions where national health examinations were conducted from 2019 to September of this year. As a result of the inspection, 593 places (2.1%) were judged inadequate. As a result, the hygiene and management of endoscope instruments that enter the human body are on the chopping block.
Looking at the status of gastric and colonoscopy examinations by the National Health Insurance Corporation, the number of related paid examinations per year is approximately 8.15 million. Including non-coverage payments, the number is close to 20 million per year. Approximately the entire population undergoes a gastroscopy every two years and a colonoscopy every three years.
Endoscopy is a test that the entire nation undergoes, but people were not interested in endoscopy equipment or instruments. It is also true that no particular meaning is attached to the idea that medical device management is left to experts.
In general, endoscopic instruments are said to be disinfected before being used again. In particular, endoscope instruments use disinfectants to manage hygiene, and surprisingly, it was found that all disinfectants used in domestic hospitals and clinics are multi-use. Endoscopic instruments that had entered another person
Voices for strengthening management and supervision of ‘endoscopic instrument disinfection’… “Uncomfortable and concerned about cross-infection”
The fact that reused disinfectant has been used to disinfect endoscope instruments is itself shocking, but it is also pointed out that government regulations are restrictive. The notice only contains regulations on the type of endoscope disinfectant, exposure time, and effective disinfection concentration. In detail, the effective concentration of the disinfectant is tested once a day, and products that do not maintain the effective concentration or have exceeded the number of reuses or expiration date are discarded. If the effective concentration of the disinfectant is judged to be appropriate, it is announced as reusable. However, the fact that endoscopic instruments washed with reused disinfectant are inserted into the human body is still uncomfortable and unpleasant. Questions may arise about the management status of hospitals and clinics, and concerns about cross-infection are also raised. The fact that there is no specific standard for assessing the cleanliness of the disinfectant other than the effective concentration also adds to anxiety.
An industry official said, “If an endoscopic instrument that has not been properly disinfected enters the body, mucus, body tissue, viruses, bacteria, and germs on the surface of the instrument remain, resulting in various cross-infections such as hepatitis C, esophagitis, blood infection, gastric ulcer, and tuberculosis. “There is a possibility of exposure,” he pointed out. At the National Assembly inspection last month, Rep. Baek Jong-heon also ordered the government, including the Ministry of Health and Welfare, to establish guidelines for reuse and disposal of endoscope disinfectant and strengthen management and supervision of disinfection status to this effect.
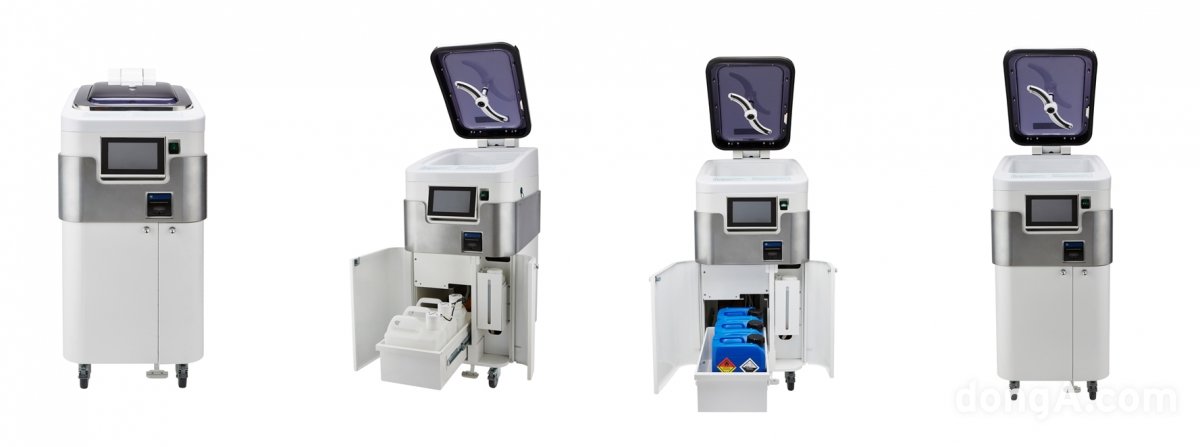
WIGP Pharmaceutical “There is no cost burden and there are many benefits in converting disinfectants from multi-use to disposable”
WIGP Pharmaceutical believes that the demand for disposable endoscope disinfectants will increase in the future as the public learns things they did not know before. It is said that disposable endoscope disinfectants were previously highly dependent on imports. However, it
Park Seong-jun, co-representative of WIGP Pharmaceuticals, said, “Use of disposable disinfectant solution can fundamentally prevent cross-infection because the endoscope is disinfected one-time each time, and there is no precipitation of contaminants in the disinfectant storage tank or endoscope equipment.” He added, “Check the effective concentration or disinfection power. “The strip paper is fundamentally unnecessary, and above all, it can eliminate hygiene-related concerns by greatly suppressing the creation of biofilm, which can enter the human body and cause abdominal pain and colitis,” he explained.
It is said that the disposable disinfectant Wickrean PA 15 liquid ultimately decomposes into acetic acid, water, and oxygen after use. WIGP Pharmaceutical said that it can be expected to be eco-friendly and reduce the burden of medical waste disposal. In terms of cost, the dedicated sterilizer CYW-S100 is priced about 3 million won higher than the multi-use sterilizer products currently used on the market, but it is said that the cost burden can be reduced through leasing or rental. In the case of disinfectant solution, 5 liters of high-concentration Weaklean PA 15 solution is needed for 60 disinfection operations, and the existing multi-use disinfectant solution requires about 8 liters, but since the prices are similar, additional costs are limited.
Kang Jin-gu, co-representative of WIGP Pharmaceuticals, said, “It is necessary to change to a disposable disinfection system to eliminate concerns about cross-infection during endoscopy and to conduct healthier endoscopy,” adding, “In the past, reusing disinfectant was the only way, but it was produced domestically with domestic technology. “Now that a disposable disinfectant solution has been released, we hope that many people will be able to enjoy the benefits associated with it,” he said.
-
- great
- 0dog
-
- I’m sad
- 0dog
-
- I’m angry
- 0dog
- I recommend it
- dog
Hot news now
What are the health risks associated with reusing disinfectants for endoscopic instruments?
The use of reused disinfectant for endoscopic instruments has raised significant health concerns regarding cross-infection. While government regulations specify certain criteria for disinfectant use—such as type, effective concentration, and exposure duration—these standards do not address the cleanliness or adequacy of the disinfectants beyond their concentration levels. This oversight is alarming, considering the potential for cross-infection from pathogens remaining on improperly disinfected instruments.
Industry experts express that improperly disinfected instruments can transmit serious infections, including hepatitis C and tuberculosis. In light of these risks, calls for stricter government guidelines regarding the reuse and disposal of disinfectants have intensified, particularly highlighted during recent National Assembly inspections.
In response to these challenges, WIGP Pharmaceutical advocates for disposable endoscope disinfectants as a safer alternative. They claim that using single-use disinfectants can significantly lower the risk of contamination, as each use would involve a fresh solution, eliminating any potential for harmful residue. The company also emphasizes the eco-friendliness of their solution, Wicklean PA 15, which decomposes into harmless substances post-use.
Despite the initial higher cost of the dedicated sterilization equipment, WIGP argues that the financial impact can be mitigated through leasing options, and overall operational costs may remain comparable to existing multi-use systems. With advancements in domestic manufacturing, the hope is that adopting disposable disinfection systems will enhance the safety and efficacy of endoscopic procedures, thereby reassuring patients and medical professionals alike about the hygiene of medical instruments.

