Domestic and foreign pharmaceutical companies are actively researching ADCs
A game changer in the treatment of incurable cancer… Market forecast worth 39 trillion won in 2028
The price barrier is high, but health insurance is not applicable… Support for terminally ill patients in the UK and Taiwan
“We need to be flexible to extend life.”
The growth of ‘antibody-drug conjugates (ADCs)’, a type of ‘guided missile’ that catches cancer cells, is rapid. According to global market research firm Evaluate, the ADC market size has grown tenfold in eight years from approximately $1 billion (approximately KRW 1.4 trillion) in 2015 to approximately $10 billion (approximately KRW 14 trillion) in 2023. It is expected to grow to $28 billion (approximately 39 trillion won) by 2028.
There are more than 150 ADC-related clinical trials currently underway worldwide. In this atmosphere, global pharmaceutical companies such as Gilead Sciences, AstraZeneca, and MSD, as well as domestic pharmaceutical companies such as Lego Cam Bio, Dong-A ST, and Genome & Company, are jumping into ADC development one after another.
● ‘Game changer’ in cancer treatment
In the past, ‘cytotoxic anticancer drugs’, which were anticancer drugs, had an excellent ability to destroy cells, but had the problem of attacking normal cells as well. ADC is a treatment product in which a toxic drug (payload) that destroys cells is attached to an antibody as a conjugate (linker). When the antibody binds to cancer cells, the toxic drug is separated and destroys the cancer cells. ADC adds precise targeting ability to cytotoxic anticancer drugs, delivering a strong cytotoxic effect only to cancer cells and minimizing the effect on normal cells.
ADC is playing a game-changing role in the treatment of various incurable cancers that are difficult to treat and have poor prognosis. A representative disease is ‘triple negative breast cancer’. For triple-negative breast cancer, cytotoxic anticancer drugs with severe side effects have been used as standard treatment. However, recently, when the ADC anticancer drug ‘sacituzumab govitecan’ was injected into metastatic patients, the effect of prolonging survival was found to be better than that of cytotoxic anticancer drugs. Since then, sacituzumab govitecan has established itself as a standard treatment for patients with secondary or higher metastatic disease.
In addition, the ADC anticancer drug ‘Enpotumab vedotin’ became the first-line treatment for ‘metastatic bladder cancer’, which had no treatment other than cytotoxic anticancer drugs. Gemtuzumab ozogamycin, an ADC anticancer drug for acute myeloid leukemia, is also used in developed countries such as the United States and Europe.
● It is expensive and not covered by health insurance.
<figure class="img_cont
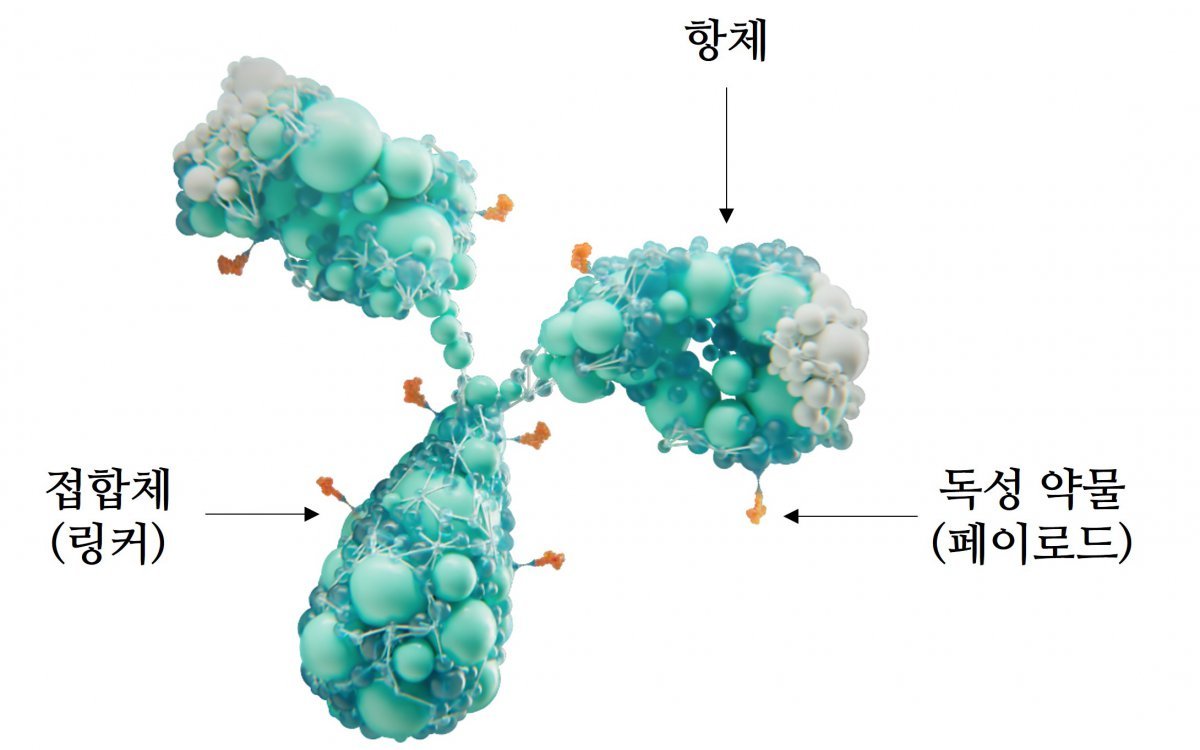
ADC anticancer drugs do not show any significant toxicity when introduced into the human body, but release toxic drugs when antibodies bind to cancer cells. To achieve this, three elements must be harmonized: an antibody that can accurately target and penetrate cancer cells, a toxic drug with a strong cytotoxic effect, and a linker that can connect them and release the toxic drug at the appropriate time. Unlike anticancer drugs that consist of only one antibody, the conjugation method and ratio of the three elements, post-conjugation reaction, and safety must be considered. Development costs are high and the manufacturing process is complex, making it difficult to produce with consistent quality. This is why the price of anticancer drugs is bound to become expensive.
The high price of ADC anticancer drugs is a realistic barrier to patients. Technological innovation has overcome the limitations of existing anticancer drugs, but the drugs are expensive and not yet covered by health insurance.
In order for patients to receive health insurance benefits, it must be passed by the Cancer Disease Review Committee, which evaluates the effectiveness of drugs and determines insurance benefits, and the Pharmaceutical Benefits Evaluation Committee, which determines treatment prices and insurance coverage. Sacituzumab govitecan received a retrial from the Pharmaceutical Reimbursement Evaluation Committee in August of this year, and enpotumab vedotin was passed by the Cancer Disease Review Committee, but has not even been submitted to the Pharmaceutical Reimbursement Evaluation Committee for the next nine months. Gemtuzumab ozogamycin was rejected three times by the Pharmaceutical Reimbursement Evaluation Committee.
● “Apply flexible health insurance considering terminally ill patients”
When deciding whether to apply health insurance for a new drug, the cost required to extend life is compared with existing drugs. If the government determines that it is difficult to cover the cost of a new drug, it will not receive a good score in health insurance adequacy. As a result, it is difficult for new drugs that have excellent life-extending effects but are expensive to receive good scores in economic evaluations.
To overcome this dilemma of new drugs, major developed countries are applying various economic evaluation standards when registering them for health insurance, or applying them first and then evaluating them afterwards. Most ADC anticancer drugs are essential for patients with incurable cancer with limited treatment options, which is why insurance coverage is urgent. An official from the medical industry said, “ADC anticancer drugs used for metastatic triple-negative breast cancer were covered by health insurance in the UK and Taiwan.” He added, “In particular, in the UK, although they did not receive a good score in economic evaluation, they were covered by health insurance considering that they prolong the lives of terminally ill patients. The application was approved. “Korea also needs to take note of this,” he said.
han Lee, medical reporter and doctor [email protected]
-
- great
- 0dog
-
- I’m sad
- 0dog
- I’m angry
- 0dog
- I recommend it
- dog
Hot news now
What are the benefits of Antibody-Drug Conjugates (ADCs) in cancer treatment compared to traditional therapies?
The article discusses the transformative role of Antibody-Drug Conjugates (ADCs) in cancer treatment, particularly for challenging conditions like triple-negative breast cancer and metastatic bladder cancer. ADCs are designed to selectively deliver cytotoxic drugs to cancer cells while minimizing damage to normal cells, thereby enhancing effectiveness and reducing side effects commonly associated with traditional cytotoxic anticancer therapies.
Key points include:
- Mechanism of Action: ADCs consist of an antibody that specifically targets cancer cells, a toxic drug that kills those cells, and a linker that connects the two. The design ensures that the drug is released only upon binding to the targeted cancer cells.
- Clinical Impact: The ADC ‘sacituzumab govitecan’ has shown improved survival rates for patients with metastatic triple-negative breast cancer compared to standard cytotoxic drugs. Similarly, ‘Enpotumab vedotin’ has become a first-line treatment for metastatic bladder cancer.
- Challenges with Access: Although ADCs have revolutionized treatment options, their high cost poses a significant barrier to patient access. Currently, these drugs are often not covered by health insurance.
- Insurance and Accessibility: The process of securing health insurance coverage for these innovative treatments is complex. Drugs must undergo evaluations by committees that assess their effectiveness and worthiness for reimbursement. There have been recent attempts to review the coverage for ADCs in South Korea, but challenges remain.
- Call for Policy Change: The article advocates for more flexible health insurance policies, especially for terminally ill patients who might benefit significantly from these advanced therapies. Comparisons are made to practices in countries like the UK and Taiwan, where ADCs have been covered by health insurance despite high costs, based on their potential to prolong life for patients with limited treatment options.
while ADCs represent a significant advancement in cancer therapy, their affordability and insurance coverage remain critical obstacles that need to be addressed to ensure wider access for patients in need.

