2024-07-31 01:10:39
Joint development of novel hyaluronidase
Expanding contract from 1 biosimilar product to multiple products
Alteogen “Expanding the Items Converted to SC Formulation”
Sandoz Strengthens SC Formulation Biosimilars and Enters Market↑
CEO Park Soon-jae returned from a business trip to the US last week… Details not disclosed
Bioplatform company Alteogen announced on the 30th through a public disclosure that it has signed a technology export agreement with Swiss global pharmaceutical company Sandoz for the development of a new hyaluronidase and a number of biosimilar products using it. This agreement will replace the existing agreement signed on December 29, 2022.
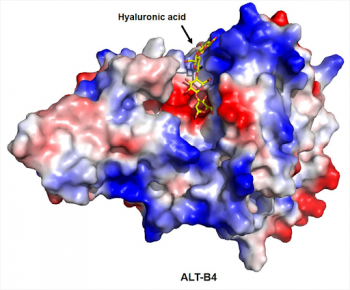
In 2022, Alteogen and Sandoz signed a technology export agreement to apply ‘ALT-B4’, a hyaluronidase-based subcutaneous injection (SC) formulation conversion technology (substance) developed by Alteogen, to one biosimilar product. At the time, the contract fee and milestone were reported to be approximately KRW 200 billion. With this contract, the existing ALT-B4 contract was decided to be terminated.
Alteogen explained that this contract was pursued in accordance with the business strategy to strengthen the hyaluronidase pipeline and increase applicable biopharmaceutical items. Under the new contract, Sandoz will be able to develop multiple SC formulation biosimilars rather than just one item using the newly developed hyaluronidase-based SC formulation conversion technology. Alteogen will receive staged milestones and separately receive post-marketing sales royalties. The specific contract terms and development strategies have been agreed not to be disclosed.

Sandoz expects this agreement to have a positive impact on strengthening its specific SC formulation biosimilar pipeline and facilitating smooth market entry for future biosimilar products.
An Alteogen official said, “Compared to the existing contract, we have expanded the scope of target items, so we expect to strengthen our partnership with Sandoz and further increase Alteogen’s presence in the SC formulation biosimilar market,” adding, “We will do our best to become a bio company with the best technology in the hyaluronidase field.”
Alteogen is a bioplatform company that has recently attracted the attention of global pharmaceutical companies. It possesses the ‘Hybrozyme’ platform technology that changes intravenous injection-type treatment into SC treatment using hyaluronidase, the ‘NexP’ platform that increases the persistence of drugs in the body, and the antibody-drug conjugate (ADC) platform ‘NexMab’. Recently, Tergase, a hyaluronidase ALT-B4 product, received product approval from the Ministry of Food and Drug Safety and is preparing for commercialization. Hyaluronidase products are used in various fields such as orthopedics and neurosurgery to facilitate drug absorption or relieve pain. Tergase is characterized by being a human-derived hyaluronidase, differentiating it from existing animal-derived products. It has significantly reduced side effects such as allergies, and various treatments can be developed using Tergase in the future.
The Hybridzyme platform, which is a SC formulation conversion technology, is being used to develop the SC formulation of the blockbuster immunotherapy drug ‘Keytruda’ developed by Merck (MSD) in the US. Currently, the related phase 3 clinical trial is in progress, and the royalty that Alteogen will receive in relation to commercialization is receiving much attention. Keytruda was the product that ranked first in global pharmaceutical sales last year, with global sales reaching tens of trillions of won. Alteogen also expects that its profits will naturally increase significantly when Keytruda SC is launched on the market. In addition, technology export contracts related to SC formulation conversion have been signed with other global pharmaceutical companies.
Meanwhile, according to the industry, Park Soon-jae, CEO of Alteogen, recently went on a business trip to the United States and returned to Korea last Saturday. The details of CEO Park’s business trip, such as its schedule and content, have not been disclosed, but since Alteogen has recently been strengthening its cooperation with global big pharmas, the industry is also interested in the details of the business trip.
Kim Min-beom, Donga.com reporter [email protected]
-
- great
- 0dog
-
- I’m so sad
- 0dog
-
- I’m angry
- 0dog
-
- I recommend it
- dog
Hot news right now
2024-07-31 01:10:39

