Dhaka, Bangladesh – February 28, 2025 – Four confirmed cases of Nipah virus, a frighteningly lethal zoonotic disease, were reported in Bangladesh in 2025, tragically resulting in four deaths. That’s a case fatality rate of 100% for these recent infections, raising concerns among public health officials.
A Resurgent Threat: Nipah Virus Cases Surface Across Bangladesh
The highly contagious Nipah virus continues to pose a important public health challenge, with recent cases appearing in geographically diverse regions of Bangladesh.
- Nipah virus (NiV) has a high fatality rate, frequently enough exceeding 70%.
- The virus is typically transmitted through contaminated palm sap or close contact with infected individuals.
- A Phase II clinical trial for a Nipah virus vaccine is underway in Bangladesh,offering a glimmer of hope.
- Recent cases have occurred outside the typical December-April outbreak season, prompting examination.
What exactly *is* Nipah virus, and why should we be concerned? Nipah virus (NiV) is a rare but incredibly risky viral disease that jumps from animals to humans. It’s known for it’s high case fatality rate-often 70% or higher-and can cause severe respiratory illness and encephalitis (brain inflammation).
Recent Cases and Geographic Spread
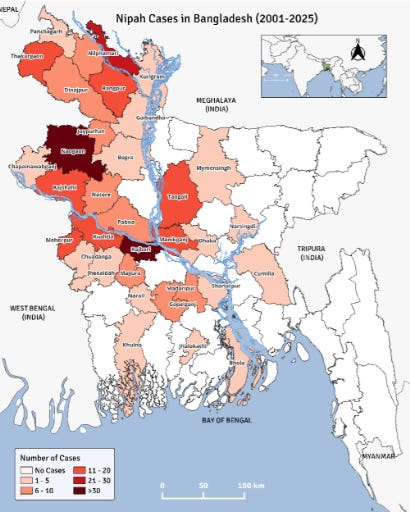
The Institute of Epidemiology Disease Control and Research (IEDCR) in Dhaka reported the four confirmed cases occurred in distinct districts across three divisions: Barisal, Dhaka, and Rajshahi. This scattered distribution suggests the virus might potentially be spreading beyond its typical hotspots.
The second case, an adult man from Bhola district, Barisal division, succumbed to the virus on February 22 after becoming ill on February 13. A third adult man from Faridpur district, Dhaka division, died on February 25, just one day after symptom onset on February 17.
The trial, which began earlier this month, will enroll 306 healthy participants aged 18 to 55.The vaccine, known as ChAdOx1 NipahB, utilizes the same viral vector platform as the oxford/AstraZeneca COVID-19 vaccine. First-in-human trials, involving 51 participants in Oxford, have shown promising results, with one year of follow-up completed as of January 2024.
Dr. Kent Kester, CEPI’s Executive Director of Vaccine Research and Advancement, emphasized the importance of this trial: “Oxford’s Nipah virus vaccine candidate is the most advanced vaccine against this highly lethal virus. The start of this phase II trial is a first of its kind and represents the culmination of years of cutting-edge research and global scientific collaboration.”
The development of a Nipah virus vaccine represents a crucial step forward in protecting vulnerable populations from this devastating disease. While challenges remain, the ongoing research offers a beacon of hope in the fight against Nipah virus.