Using advanced methods for genetic sequencing at the single cell level, scientists at the Weizmann Institute of Science unveil a new molecular mechanism of action of ketamine – and show that its combination with another drug may lead to improved performance and reduced side effects
Readers of the magazine TIME Announced in July 2017 in the front page article on “New hope for depression”: Low dose of ketamine, a known anesthetic used in operating rooms – and also in clubs as a drug for subconscious reasons – has been shown in a number of studies as”A magic potion” For those who suffer from suicidal thoughts. Two years later, in March 2019, the US Food and Drug Administration (FDA) approved the first drug to treat depression based on ketamine – a nasal spray from Johnson & Johnson.
Although this is the most significant development in the field of depression treatment for decades, the drug is currently approved for patients with persistent depression who do not respond to other treatments. The reasons for this, among others, are the fact that this is a new drug whose mechanisms of action are not yet sufficiently understood and the fear of possible side effects. In a new study published today in the scientific journal NeuronScientists from the Weizmann Institute of Science reveal new details, at the level of the single cell, about the mode of action of ketamine and point out possible directions for the safe and effective treatment of depression.
The rate of those suffering from clinical depression is on the rise in developed countries, including Israel; Two years of international epidemic did not help improve statistics. Despite the alarming numbers, the economic cost and the damage to human life, there has been no significant breakthrough in the field of depression treatment for many decades; Certainly not since the most famous antidepressant drug, Prozac, was approved for use in 1987. In recent years, there have been increasing questions about the effectiveness of existing treatments for depression, to which a third of patients do not respond at all.
Another notable disadvantage of existing treatments is the prolonged time it takes for them to take effect – four to eight weeks; In patients with suicidal tendencies, such a gift is a matter of life and death. Precisely for this reason, the introduction of ketamine-based treatments is considered a breakthrough: their antidepressant effect is extremely rapid – a few hours. Moreover, the antidepressant effect continues even after the drug itself has been cleared from the body. In other words, the body’s response to ketamine, more than ketamine itself, is responsible for the antidepressant effect. But what is that reaction? There has been no clear answer to this so far, and it is possible that they simply did not look for it in the right way.

Previous studies that have attempted to crack the mechanism of action of ketamine have examined the effect of the drug on gene expression in the brain, but not at the level of the single cell but at the level of tissue. When gene expression is examined in a tissue sample that includes cells of different types, possible differences between cells are deleted and may screen for significant effects.
Technological developments of recent years allow scientists to measure the expression of genes in tissue at the individual cell level, thus characterizing at unprecedented resolution differences between cells in the same tissue. In the current study, a research team in the laboratory of Prof. Alon Chen – a joint research laboratory for the Weizmann Institute of Science and the Max Planck Institute – led by the postdoctoral researcher, d”R. Juan Pablo Lopez, used these methods to comprehensively map the expression of genes in thousands of neurons in mice given a dose of ketamine.
Molecular chain reaction
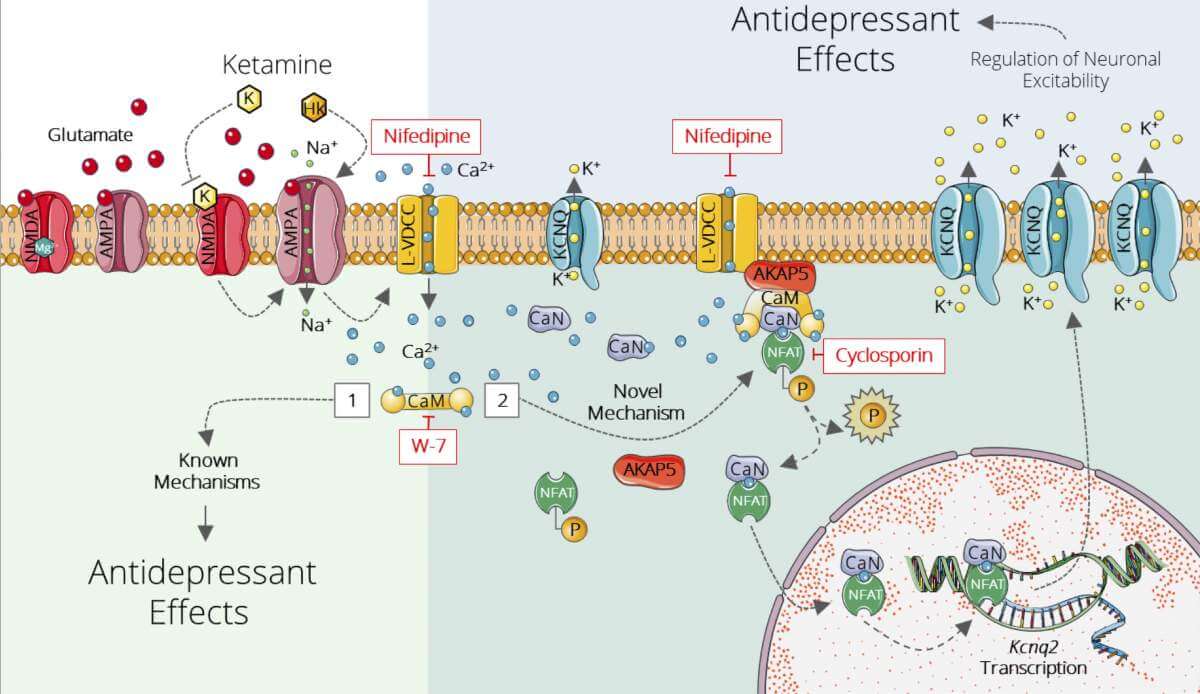
As early as the 1990s it was discovered that unlike the old drugs that act mainly on neurons affected by the neurotransmitter serotonin, ketamine affects neurons of the glutamate system – another major neurotransmitter. However, given that the antidepressant effect remains long after the drug is cleared from the body, the mechanism of action cannot be attributed solely to the direct ability of ketamine to block one of the receptor types of nerve cells to glutamate.
“We wanted to understand the molecular chain reaction that occurs following taking ketamine, with the understanding that it is this reaction that produces the long-term effect”Explains d”R. Lopez. To this end, the researchers focused on the anterior hippocampus – an area known from previous studies as an important site when it comes to the antidepressant effects of ketamine.
When the researchers mapped the genetic expression of the cells in this tissue in mice, they discovered differences in the genetic expression between subpopulations of nerve cells that have hitherto disappeared from the eyes of scientists. The most significant genetic marker found in them was in a subpopulation of cells that showed increased expression of Kcnq2; This gene encodes potassium channels, that is, channels on the cell membrane that open as a result of a change in electrical voltage and allow the passage of potassium ions. These channels play a key role in nerve cells, and are known to be involved in maintaining nerve balance and inhibiting cell overactivity. Through a series of experiments in mice – at the molecular and cellular level and through behavioral, pharmacological, functional and electrophysiological tests – the scientists showed that this genetic seal is indeed involved in the antidepressant effect of ketamine.
Following the findings, the scientists tried to test what would happen to the effect of ketamine if they added another drug, retigabine, which is known to activate potassium channels in the brain and is currently given to epilepsy patients. The researchers found that the combination of drugs significantly strengthens the antidepressant effect of ketamine. “A single dose of retigabine was sufficient to increase and prolong the antidepressant effects of ketamine in mice”Says d”R. Lopez. “Moreover, the combination of drugs has led to a similar effect even at lower doses of ketamine and may allow for the reduction of unwanted side effects.”. Because the two drugs have already received FDA approval, the way is paved to test the effect of the combination between them on humans in clinical trials.
Hundreds of millions of people around the world suffer from depression, and hundreds of thousands commit suicide every year. Even after decades of research, this life-threatening disorder is not sufficiently understood. “Deepening the understanding of the mechanisms by which antidepressants work is one of the ways to better understand depression – as well as to improve existing treatments”, Prof. Chen explains that the research team led by him has unveiled a new mechanism of action of ketamine – the most promising drug today in the field of depression treatment. However, despite the great promise, the use of ketamine is limited due to the lack of sufficient information. It is possible that the new findings will help expand the use of ketamine-based therapies – whether alone or in combination with other drugs – and thus more fully fulfill the new hope for people suffering from depression.
