Selected as the 2nd national new drug development project in 2024
Supports approximately 600 million won in research funds from the National New Drug Development Foundation
“Pointing out the effectiveness of the scale of support for the National New Drug Development Foundation”
Goal of developing the world’s first ‘oral new drug’
Mechanism of inhibiting collagen accumulation that causes liver fibrosis
Daewoong Pharmaceutical is beginning the development of its first oral treatment for severe liver fibrosis.
Daewoong Pharmaceutical announced on the 18th that its new drug candidate ‘DWP220’ was selected as a project for the 2nd National New Drug Development Project in 2024 hosted by the Korea New Drug Development Foundation (KDDF).
The National New Drug Development Project is a pan-ministerial national R&D project launched to strengthen the global competitiveness of the domestic pharmaceutical and bio industry. For 10 years from 2021, we will support the entire cycle of new drug development with the goals of strengthening the domestic R&D ecosystem for new drug development, creating global commercialization results, and creating public benefits in the health and medical field.
However, the actual scale of research funding for the national new drug development project is assessed to be insignificant compared to the grand project purpose. Depending on the project, the total research budget is set at 800 million to 7 billion won, and the government supports 50% of each project. In fact, the amount of government support is in the range of 400 to 3.5 billion won. Considering that the cost of developing new drugs generally ranges from tens of billions of won to hundreds of billions of won, and that the development period is long, the content and scale of support are criticized as inadequate. In particular, logistics costs, wages, and prices have risen significantly over the past three to four years, but the size of the subsidy has remained the same.

In this national new drug development project, Daewoong Pharmaceutical’s DWP220 was selected as a candidate material (development stage) development project in the ‘Research on building a new drug R&D ecosystem’ section of the notification unit (RFP name). This project is set to have a total research budget of approximately 1.2 billion won, and 50% of this, or approximately 600 million won, is expected to be supported. Prior to this, DWP216, an anticancer drug candidate, was also selected as a non-clinical development project for the same item in this national new drug development project. The total research budget is around 2 billion won, which is slightly more than DWP220.
DWP220 has a mechanism to inhibit collagen accumulation, a major factor causing liver fibrosis. In liver fibrotic disease, extracellular matrix (ECM) components, especially collagen, accumulate excessively, causing the tissue to become hard and its function to decrease. DWP220 aims to prevent the progression of fibrosis by inhibiting the production of collagen, a major component of ECM, while reducing already advanced fibrosis and alleviating tissue damage.
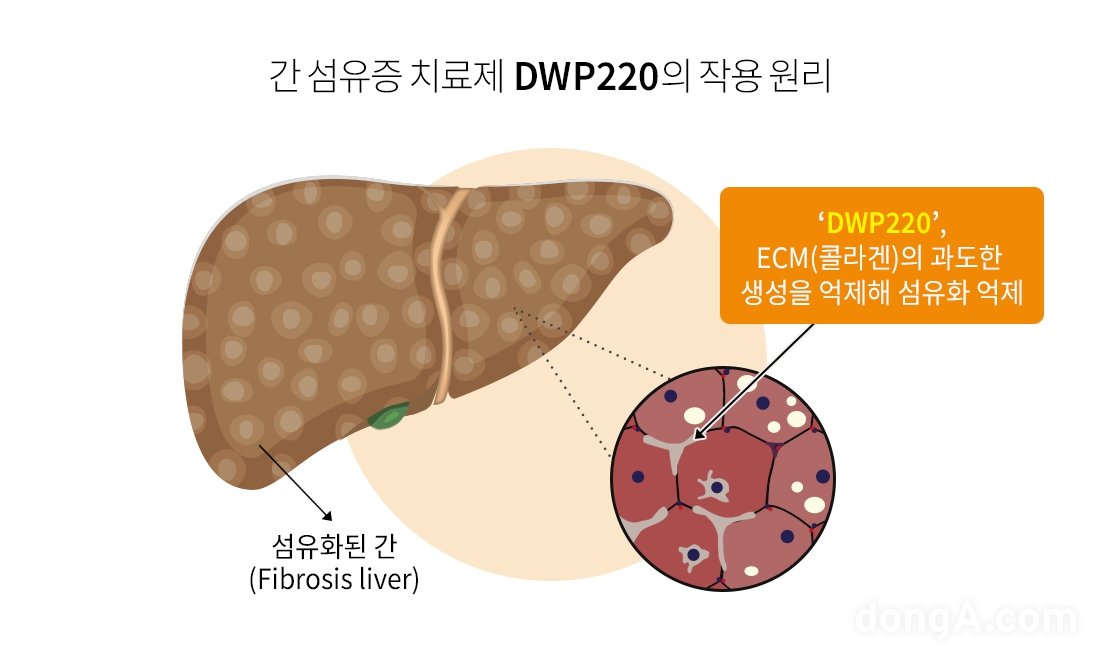
Liver fibrosis is a process in which normal liver tissue is replaced by abnormal connective tissue as repetitive damage and inflammation occurs in the liver. In general, if ‘fatty liver’, in which excessive fat accumulates in the liver, persists for a long time, it can lead to liver fibrosis.
Resmetirome, a ‘metabolic steatohepatitis (MASH) treatment’ approved by the U.S. Food and Drug Administration (FDA) this year, is assessed to have limitations in treatment as the effect of improving fibrosis was only a 1-stage improvement as a result of clinical trials. Accordingly, related experts are raising the need for further development of liver fibrosis treatments. Unmet medical needs remain high due to increased patient mortality due to worsening fibrosis.
In particular, there is no treatment that can provide fundamental therapeutic effects for severe liver fibrosis, so if DWP220 is commercialized, it is expected to become the world’s first oral treatment capable of treating severe liver fibrosis. The global liver fibrosis market is expected to grow by more than 10% annually until 2028, reaching approximately 36 trillion won.

Daewoong Pharmaceutical has secured the technical knowledge and clinical know-how necessary to develop a collagen-targeting fibrosis treatment based on its experience in developing the idiopathic pulmonary fibrosis treatment ‘Versiporosin’, which is currently in the phase 2 clinical trial stage. Based on this, the plan is to pursue the development of treatments for fibrotic diseases in a rapid and optimized manner in this project. The goal is to complete candidate material development by 2026.
Park Seong-soo, CEO of Daewoong Pharmaceutical, said, “The selection of the national new drug development project was an opportunity to reaffirm the possibility of developing a treatment for severe liver fibrosis,” and added, “We will further strengthen our capabilities in developing treatments for fibrosis and prove Daewoong Pharmaceutical’s ability to develop new drugs.” .
Kim Min-beom, Donga.com reporter [email protected]
-
- great
- 0dog
-
- I’m sad
- 0dog
-
- I’m angry
- 0dog

