2024-08-02 04:24:32
The world’s first once-monthly subcutaneous treatment
Relieves inconvenience of existing 2-week intravenous injection treatment
“Expect long-term efficacy superior to existing treatments”
GC녹십자 announced on the 1st that it has applied for an Investigational New Drug (IND) for phase 1/2 clinical trials for ‘LA-GLA (GC1134A, HM15421),’ a Fabry disease treatment being jointly developed with Hanmi Pharmaceutical, to the U.S. Food and Drug Administration (FDA).
LA-GLA is the world’s first monthly subcutaneous injection, and is a groundbreaking new drug for the treatment of Fabry disease jointly developed by GC녹십자 and Hanmi Pharmaceutical. Fabry disease is a rare disease inherited via sex chromosomes. It is a type of lysosomal storage disease (LSD). It occurs when the enzyme ‘alpha-galactosidase A’, which breaks down glycolipids in lysosomes, an organelle within cells that removes unnecessary substances, is deficient. It is known as a rare, progressive, incurable disease in which unprocessed glycolipids continuously accumulate, causing cytotoxicity and inflammatory reactions, gradually damaging various organs and, in severe cases, leading to death.
Currently, Fabry disease patients are treated with enzyme replacement therapy (ERT), which is an intravenous injection of an enzyme developed through genetic recombination technology. However, ERT treatment has the inconvenience of requiring long-term intravenous injections at a hospital once every two weeks. The burden of treatment is high, and the efficacy in suppressing progressive kidney disease is also insufficient.
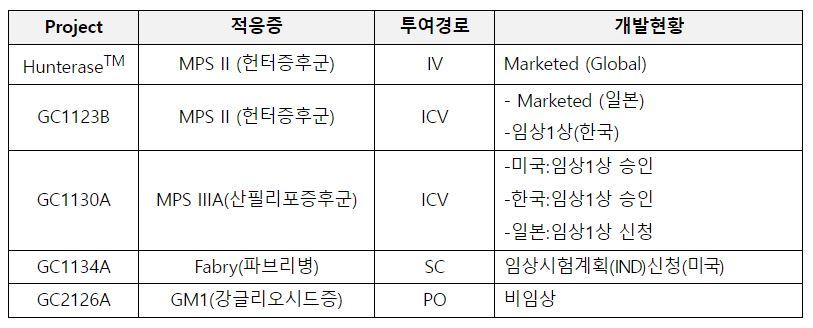
LA-GLA is characterized by resolving the limitations of existing treatments by focusing on these inconveniences. It can be considered a next-generation sustained enzyme replacement therapy treatment. In addition, it was designated as an orphan drug (ODD) by the US FDA in May based on its excellent efficacy, such as improvement of renal function, vascular disease, and peripheral nerve disorders compared to existing treatments in the non-clinical stage.
A GC녹십자 official said, “We will begin full-scale global clinical trials, starting with the U.S. and including Korea,” and added, “Based on our experience and know-how in developing treatments for lysosomal storage diseases, we will do our best to develop new drugs so that we can provide innovative treatment options to patients suffering from Fabry disease.”
Kim Min-beom, Donga.com reporter [email protected]
-
- great
- 0dog
-
- I’m so sad
- 0dog
-
- I’m angry
- 0dog
-
- I recommend it
- dog
Hot news right now
2024-08-02 04:24:32

