GC녹십자·한미약관, Joint Development of Fabry Disease Treatment ‘LA-GLA’
Approval of clinical trial plan (IND) within one month of application
World’s first monthly subcutaneous injection developed… Treatment convenience↑
Attention as a next-generation Fabry disease treatment… Designated as an orphan drug
Previous intravenous injection every 2 weeks → Subcutaneous injection once a month
GC녹십자 announced on the 2nd that its Fabry disease treatment drug ‘LA-GLA (development code name GC1134A, HM15421)’, which it is jointly developing with Hanmi Pharmaceutical, has received approval for the clinical phase 1/2 trial plan (IND) from the U.S. Food and Drug Administration (FDA). Approval came just one month after filing the IND on August 1st, allowing it to begin clinical trials in earnest.
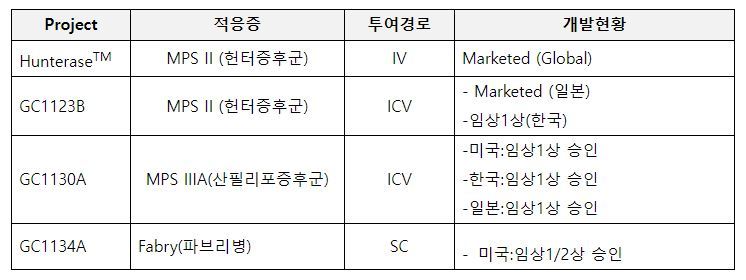
LA-GLA is the world’s first monthly subcutaneous injection, and is a groundbreaking new drug for the treatment of Fabry disease that GC녹십자 and Hanmi Pharmaceutical are jointly developing. Fabry disease is a rare, progressive, incurable disease inherited via sex chromosomes and is a type of lysosomal storage disease (LSD). It occurs when the enzyme ‘alpha-galactosidase A’, which breaks down glycolipids in lysosomes, an organelle within cells that removes unnecessary substances, is deficient. It is a rare, progressive, incurable disease in which unprocessed glycolipids continuously accumulate, causing cytotoxicity and inflammatory reactions, which gradually damage various organs and, in severe cases, death.
Currently, most Fabry disease patients are treated with enzyme replacement therapy (ERT), which is an intravenous injection of enzymes developed using genetic recombination technology. This first-generation treatment method has the inconvenience of requiring long-term intravenous injections at a hospital every two weeks. In addition, there are limitations such as the burden of treatment due to intravenous infusion and the lack of efficacy in suppressing progressive kidney disease.
LA-GLA is attracting attention as a next-generation sustained enzyme replacement therapy treatment that improves the limitations of existing treatments. The clinical trial approved this time focused on evaluating the safety, tolerability, pharmacokinetics, and pharmacodynamics of LA-GLA for patients with Fabry disease.
If the innovative new drug LA-GLA is successfully developed, it is expected to significantly improve the convenience of treatment for patients and medical staff with a monthly subcutaneous injection therapy. Its excellent efficacy in improving kidney function, vascular disease, and peripheral neuropathy compared to existing treatments has also been confirmed through non-clinical studies. In particular, LA-GLA was designated as an orphan drug (ODD, Orphan Drug Designation) by the US FDA in May in recognition of its potential for efficacy and innovation.
A GC녹십자 official said, “We were able to quickly enter the clinical stage by reflecting the latest clinical protocol required by the FDA and concentrating the specialized technologies of GC녹십자 and Hanmi Pharmaceuticals in collaboration,” adding, “Based on our experience, knowledge, and know-how in developing treatments for lysosomal storage diseases, we will do our best to develop a new drug so that we can provide a new treatment option for patients suffering from Fabry disease.”
Kim Min-beom, Donga.com reporter [email protected]
-
- great
- 0dog
-
- I’m sad
- 0dog
-
- I’m angry
- 0dog
-
- I recommend it
- dog
Hot news right now
2024-09-03 08:02:38

