After the European Medicines Agency (EMA), it is the turn of the High Authority for Health (HAS) this Tuesday, September 20, to authorize new generation vaccines against Covid-19 adapted to the Omicron variant. A recall campaign coupled with the flu vaccination should begin on October 18. This campaign will take place even though no clinical trial on these new vaccines or on the concomitant administration of the two vaccines has been undertaken or completed.
Vaccination against Covid-19 coupled with that of the flu
From the fall, part of the French will be called upon to renew their vaccination against Covid-19. New generation vaccines, more adapted to the Omicron variant, will be deployed on the European market in the fall of 2022. Approved by the European Medicines Agency (EMA) and by the High Authority for Health (HAS), they are manufactured by the firms Pfizer / BioNTech, for two of them, and by Moderna for the third. All target the Omicron variant and its sublineages. These are bivalent vaccines. Two new vaccines, one from biotech Moderna, the other from pharmaceutical company Pfizer / BioNTech, both contain the RNA sequence of the Spike protein from the wild strain of Wuhan, to which has been added the RNA sequence of the variant BA.1. The other vaccine manufactured by the firm Pfizer / BioNTech also contains the RNA sequence of the Spike protein from the wild strain of Wuhan, to which the researchers added the RNA sequences of the Spike protein from the new BA sub-lines. 4 and BA.5.
These booster vaccines are recommended for “ people over 60 and adults under 60 at risk of a severe form of the disease: those with comorbidities that expose them to these severe forms, pregnant women, from the 1st trimester of their pregnancy, immunocompromised people regardless of their age, children and adolescents at high risk suffering from pathologies justifying it “, wrote the HAS in its press release which recommends “ either one of the three bivalent vaccines suitable for Omicron variants recently validated by the European Medicines Agency ».
Also affected are ” the entourage of these people (cocooning strategy) as well as the people who are in regular contact with them: professionals in the health and medico-social sector ».
In addition, the HAS insists on combining vaccination against covid with that of influenza, recalling that “ the concomitant injection of the two vaccines is possible, if it is carried out on two distinct injection sites ».
The doubts of the High Authority for Health on new vaccines
« The clinical efficacy expected for these new bivalent vaccines is at least equivalent, or even superior to that of the original monovalent vaccines, without this probable superiority being able to be currently demonstrated in real life. “Wrote the HAS in its press release.
From these few meaningful lines, we understand that the HAS has little or no sufficient solid data as to the effectiveness of these new vaccines.
Do we still need to remember that the monovalent vaccines were developed from the wild strain of Wuhan which disappeared more than two years ago, that they are considered obsolete and that many scientists have observed the ADE phenomenon (Antibody Dependent Enhancement translated into French by “facilitation by antibodies”) which can ” occur later and occur when a person is infected with another viral serotype for which the antibody-facilitated ADE phenomenon exists, which will promote infection “. This phenomenon, which began with the appearance of the Delta variant and which was amplified with the Omicron variant and its sub-lineages, can hardly be questioned, since thousands of vaccinated people have contracted the virus and some Official data, such as those regularly published in the UK, show that vaccinated people are at least as exposed as unvaccinated people to serious forms.
Elsewhere, Israel, a pioneer in Covid vaccination, has no better data to display, as shown in the recently released table below.
« As for their tolerance, which has been studied, it is identical to that of monovalent vaccines. This is why, within the framework of the additional dose recommended this fall, the HAS recommends using, preferably, a bivalent mRNA vaccine, regardless of the vaccines used previously. “, also declares the HAS.
The statement on tolerance is also problematic for several reasons. First, since the declassification of the FDA documents, we see that there are many suspicions of adverse effects on these monovalent vaccines since the start of the vaccination campaign. American and European pharmacovigilances have recorded a number of significant cases of death and side effects. And, if the imputability of the evidence has not yet been established for all cases, the very many suspicions that exist should perhaps have led the authorities to ask themselves a certain number of questions and perhaps to interrupt a campaign. time to carry out certain checks, as was the case with swine flu in the United States in 1976, when the mass vaccination program was canceled two months later after 450 cases of Guillain’s syndrome were reported -Barré and 25 deaths.
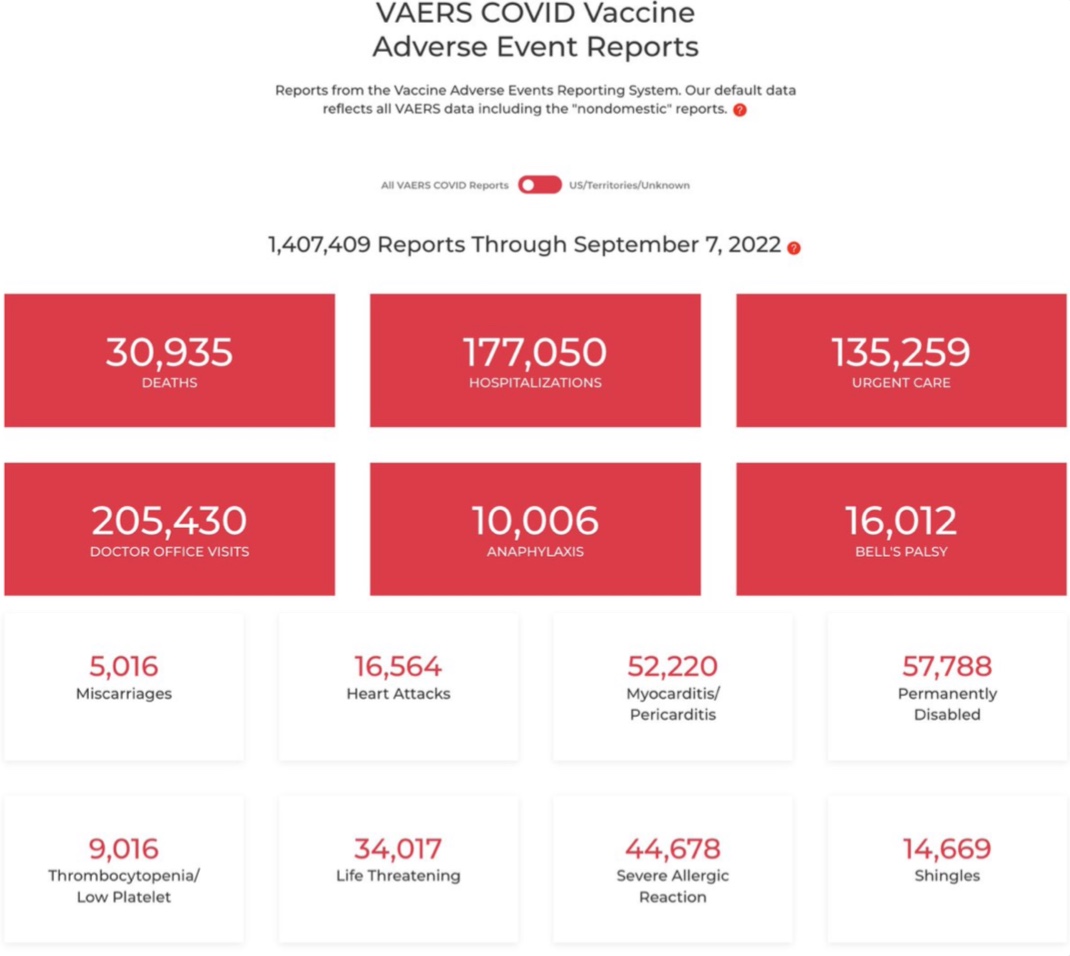
Furthermore, one is entitled to wonder how HAS can speak of an identical tolerance, even though vaccination in the general population has not started and these vaccines have not (with the exception of of a small dosage of antibodies) is the subject of clinical studies.
Vaccination in the general population without a clinical trial completed or even started
The three vaccines managed to obtain approval from the EMA and the HAS without any therapeutic trial having demonstrated that the treatment was sufficiently active and non-toxic to obtain an authorization for use, even in an emergency.
The question that remains about these approvals is how did the EMA and HAS manage to evaluate these three candidate vaccines in the absence of clinical studies carried out according to good clinical practice?
Verifying the efficacy, tolerance and safety of a new treatment or a new vaccine requires a minimum of hindsight since the people who participated in the trial must be followed up for several months, even several years before proposing new therapeutic products to the general population.
Read also: Pfizer trials on the anti-covid vaccine: the explosive report by Christine Cotton
However, as Jean-Jacques Devic explains, in the context of the two new bivalent vaccine candidates targeting BA.1: “ A few manufacturers are “testing”, or have already clinically tested the humoral immunogenic response of modified vaccines containing Wuhan + Omicron BA.1 “. These rapid studies were done to essentially monitor the level of antibodies and ” cannot be demonstrative nor guarantee job security for any new variant “, even though ” this BA.1 strain, which has disappeared since February 2022, is relatively distant at the phylogenesis level from BA.4 and 5 “. Therefore, these studies were essentially used by the FDA Vaccines and Related Biological Products Advisory (VRBPAC) Vaccine Review Committee on June 28 to make “ accept » by the American agency the bivalent gene vaccine (wild Wuhan strain + Omicron BA.1 strain).
Moreover, as can be verified by going to the clinicaltrials.gov site, two therapeutic studies on these new vaccines have been put online. Both studies concern gene therapies targeting the BA.1 variant. Examination of the Pfizer / BioNTech document shows that the study would not have started since it is indicated that the firm “cseeks new participants aged 18-55, in good health (who may have pre-existing disease if stable) “. As for that of Moderna, it would have started in June 2022 with a small cohort of 150 people. Randomized and double-blind, the final results would not be expected before June 2023.
No clinical study plans were found for the Pfizer/BioNTech vaccine candidate intended to target the BA.4 and BA.5 sublines.
The HAS recommendation to combine the Covid vaccine with the flu vaccine also raises questions. As biologist Hélène Banoun wrote in a tweet, “ this association has not been tested ».
https://t.co/wbKqKzcD4v
HAS recommends combining flu vaccine and Covid vaccine
This combination has NOT BEEN TESTED
Wild clinical trial?— Hélène Banoun (@BanounHelene) September 20, 2022
The administration of these new vaccines will therefore begin in the general population without any information in terms of medium and long-term efficacy, or even toxicity, having been the subject of any evaluation of the results with regard to the good clinical practice.
