Imfinzi Combination Therapy Demonstrates Significant Survival Benefit in Early Gastric Cancer
Table of Contents
AstraZeneca’s Imfinzi (durvalumab), in combination with chemotherapy, has shown a substantial improvement in overall survival (OS) for patients with early-stage gastric cancer, potentially reshaping treatment paradigms globally. The findings, unveiled at the European Society of Medical Oncology Annual Meeting (ESMO 2025) in Berlin on Thursday, September 17, 2025, mark a significant advancement in the fight against this challenging cancer.
Landmark Study Results from the MATTERHORN Trial
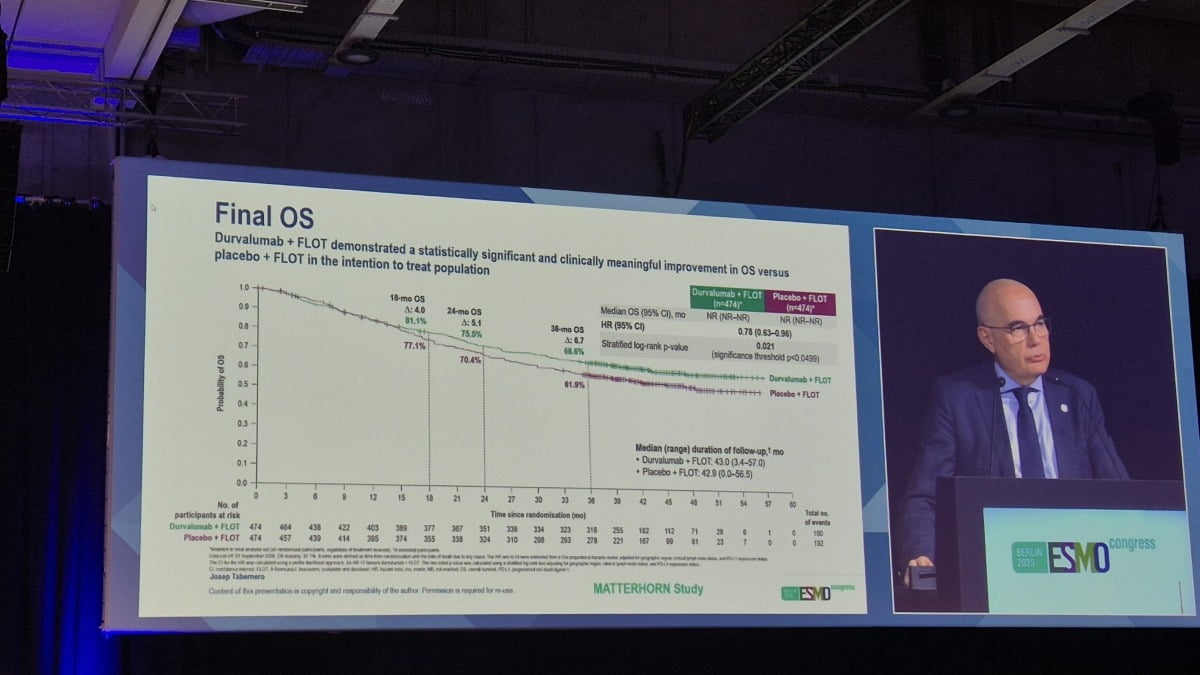
Josep Tavernero, director of the Valdevron Oncology Research Institute, presented the final OS analysis from the phase 3 MATTERHORN clinical trial. The study investigated the efficacy of combining Imfinzi with standard chemotherapy regimen FLOT (fluorouracil, leucovorin, oxaliplatin, and docetaxel) in perioperative adjuvant therapy – treatment given before and after surgery – for patients with resectable gastric cancer.
The data revealed a striking 22% reduction in the risk of death among patients receiving the Imfinzi combination compared to those treated with FLOT alone. Importantly, the benefit appeared to grow over time, suggesting a durable effect of the immunotherapy. At 18 months, the OS rate for the Imfinzi combination group was 81.1%, compared to 77.1% in the control group – a difference of 4.0 percentage points. This gap widened to 5.1 percentage points at 24 months (75.5% vs. 70.4%) and further to 6.7 percentage points at 36 months (68.6% vs. 61.9%).
“Clinically meaningful improvement in overall survival rate was observed with combination therapy,” stated Director Tavernero. “We strongly support its establishment as a new global standard treatment.”
Shifting Treatment Paradigms in Korea and Beyond
Currently, post-operative adjuvant chemotherapy is the standard of care for patients with resectable gastric cancer in Korea. However, a prevailing belief has been that pre- and post-operative adjuvant therapy is more suited for Western populations. This new data challenges that notion.
According to analysts, the success of the MATTERHORN trial, demonstrating significant clinical benefit with an immunotherapy-inclusive adjuvant approach, could represent a turning point. The results are anticipated to drive a shift towards perioperative adjuvant treatment in Korea and potentially other regions.
“This is a significant step forward,” one analyst noted. “The long-term survival data is particularly compelling and suggests that this combination therapy could fundamentally change how we approach gastric cancer treatment.”
The implications of these findings extend beyond Korea, offering hope for improved outcomes for patients worldwide battling this aggressive disease. The MATTERHORN trial’s success underscores the growing role of immunotherapy in early-stage gastric cancer and paves the way for further research exploring optimal treatment strategies.