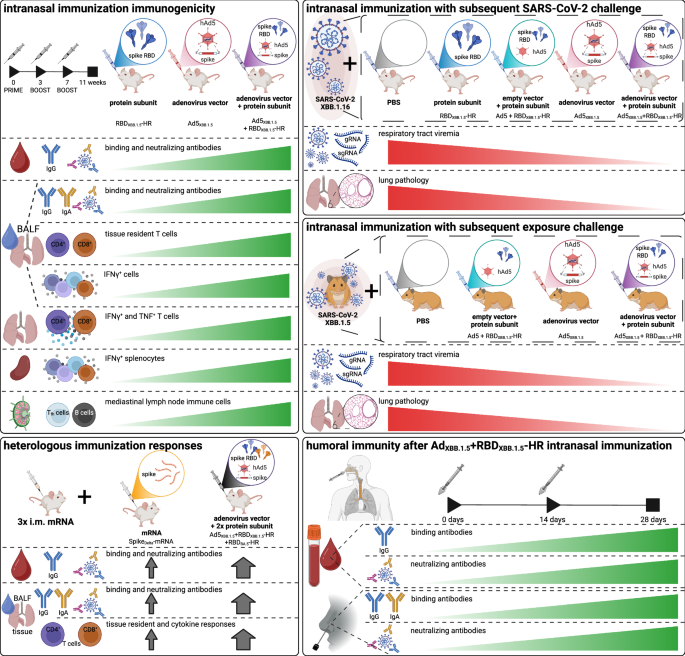
A novel, two-component intranasal COVID-19 vaccine-combining an adenovirus vector and a recombinant protein-generated both systemic and mucosal immunity, and even prevented viral transmission in animal models, according to research published in Nature Biomedical Engineering.
A New Nasal Spray Could Be a Game Changer for COVID-19 Immunity
Table of Contents
Scientists are reporting promising results for a next-generation COVID-19 vaccine delivered right where the virus enters the body: your nose.
- The vaccine uses a combination of two technologies-an adenovirus vector and a protein subunit-to boost the immune response.
- Animal studies showed the vaccine not only protected against infection but also prevented transmission of the virus.
- Human trials demonstrated the vaccine was safe and triggered robust antibody responses.
The frantic race to develop COVID-19 vaccines delivered quickly, with mRNA and inactivated virus platforms leading the charge. But a growing number of immunologists believe that delivering vaccines directly to the mucosal surfaces-like the lining of the nose-could offer a more robust and lasting defense against respiratory viruses. This new research suggests they might potentially be right.
Why Nasal Vaccines Matter
most existing COVID-19 vaccines are administered via intramuscular injection, a safe and effective method.However, this approach doesn’t always mimic the natural route of infection, potentially limiting the progress of strong mucosal immunity-the first line of defense in the respiratory tract. Mucosal vaccines, delivered intranasally or via inhalation, aim to address this by directly stimulating the immune system where it encounters the virus.
A Two-Pronged Approach to Immunity
In their recent publication,Weiqi hong and colleagues developed a two-component intranasal vaccine targeting the Omicron XBB.1.5 variant. The vaccine consists of a human adenovirus (Ad5) engineered to express the full spike protein,paired with a recombinant spike receptor binding domain (RBD) protein (RBDXBB.1.5-HR). The adenovirus acts as a delivery system, ferrying the protein into the mucosal tissues and concurrently boosting the immune response. The researchers also explored combinations targeting the BA.5 variant and both XBB.1.5 and BA.5 variants.
As of now, five mucosal SARS-CoV-2 vaccines have received limited approval globally. These vaccines utilize different platforms-protein subunit, adenoviral vector, or live attenuated influenza virus-and target components of the original SARS-CoV-2 spike protein. Focusing solely on the spike protein may not provide lasting protection against emerging variants, as mutations can allow the virus to evade antibody responses.
Vaccines incorporating both spike and non-spike proteins, like the envelope, membrane, and nucleocapsid, could offer broader and more durable immunity. The work by Hong and colleagues underscores the potential of combining adenoviral vectors with other vaccine platforms to induce robust mucosal immunity and represents a promising avenue for future vaccine development.