Obesity companies actively discover indications
Knee pain improved as a result of ‘Wegobi’ administration… ‘Zebbound’ helps with sleep apnea
As the prevalence of obesity decreases, the target appears to be expanding.
There are cases of death after increasing the dosage… “Beware of side effects such as headache, vomiting, and diarrhea.”
Research results have shown that the obesity treatment drug ‘Wegobi’ (ingredient name: semaglutide), which was recently released in Korea and is causing a shortage, is effective in treating arthritis. In addition to cardiovascular disease and Alzheimer’s, it has been recognized for its efficacy in arthritis. However, in the United States, a case was reported in which a patient in his 70s who had been taking anti-obesity medication for a long period of time died from side effects of pancreatitis after increasing the dose, and some are pointing out that we should be cautious about indiscriminate medication.
● Expansion of indications from arthritis to sleep apnea

On the 30th of last month (local time), a joint research team including the University of Copenhagen in Denmark and the University of Oslo published research results showing that Wigobi was effective in treating arthritis in the international academic journal ‘New England Journal of Medicine (NEJM).’ This research was conducted with support from Novo Nordisk, the developer of Wegobee. This is the first study to show that glucagon-like peptide-1 (GLP-1) obesity treatments such as WeGobee are effective in treating arthritis.
The research team conducted a 68-week clinical trial with 407 participants divided into a Wegobi treatment group and a placebo group. The participants were all obese and had an average score of 70.9 out of 100 on the knee pain scale due to arthritis. This is a level of pain that makes even walking painful.
The experimental group administered Wigobi for 68 weeks had an average pain scale drop of 41.7 points, while the placebo group only dropped 27.5 points. Looking at the score alone, the pain in the Wegobi treatment group was reduced by about 1.5 times more than the placebo group. The research team explained that the combination of weight loss caused by the administration of Wegobee and the anti-inflammatory effect of WeGobee appears to have significantly alleviated pain.
Previously, Eli Lilly, another leader in obesity treatment, published research results in the same academic journal in April this year showing that GLP-1 obesity treatment ‘Zebbound’ (ingredient name: tyrzepatide) is effective in improving sleep apnea. In a clinical trial in which 469 people participated, the Zebbound treatment group showed improvement in symptoms by reducing the apnea/hypopnea index (AHI) by about 55%. Based on the results of this study, the company has applied for approval as a sleep apnea treatment to the U.S. Food and Drug Administration (FDA), but the approval results have not yet been released.
● Demand for obesity treatment peaks – concerns over side effects
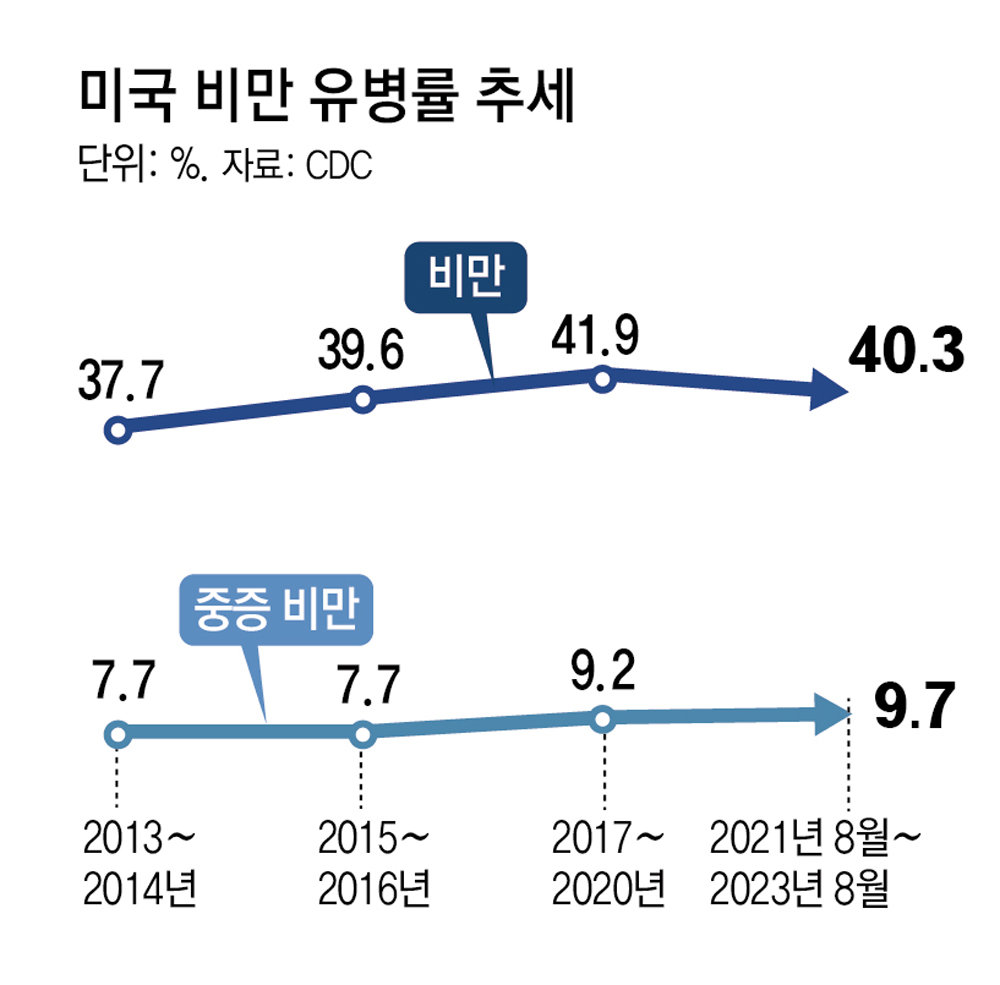
The reason obesity treatment developers are actively expanding the indications for GLP-1 treatment is because there are observations that demand from obese patients may reach its peak sooner than expected. Although 35% of adults in 23 states in the United States still suffer from obesity, data has shown that the prevalence of obesity in the United States has decreased over the past 10 years.
As the administration of GLP-1 treatment is prolonged, reports of side effects are also emerging. According to a paper published in the Science and Technology Citation Index (SCI)-level international journal ‘Cureus’ in September this year, a 74-year-old man in the United States who showed symptoms of diabetes and obesity developed acute pancreatitis after increasing the dosage of semaglutide treatment. There have been cases of death after being hospitalized. Pancreatitis is cited as one of the side effects of semaglutide. This man suffered from type 2 diabetes, coronary artery disease, and obesity (BMI 31.7) and had been taking the drug for 4 years. The dose was doubled 4 weeks before hospitalization. Afterwards, he was hospitalized with severe acute pancreatitis and died from complications. “Suggesting that there is a potential link between long-term use of GLP-1 agents and changes in dosage and severe pancreatitis,” the paper said.
Among the GLP-1 drugs, Wigobi first entered Korea and is gaining popularity, and the Ministry of Food and Drug Safety also asked people to refrain from misuse. Do Won-im, a researcher at the Ministry of Food and Drug Safety, appeared on YTN radio on the 31st and said, “Wegobi can cause side effects such as headache, vomiting, diarrhea, constipation, and acute pancreatitis.” He added, “In the United States, a 74-year-old man who increased the dose of Wegobi suffered from severe illness. “It is reported that he died of pancreatitis,” he said. In addition, recently, even non-obese people are taking anti-obesity drugs, raising concerns about misuse. “If you purchase Wegobi directly from overseas or through private transactions, there may be products that are outside of the appropriate storage temperature and have quality problems. He also said, “You must use Wigobi that is normally distributed.”
Reporter Choi Ji-won [email protected]
Reporter Kim So-young [email protected]
-
- great
- 0dog
-
- I’m sad
- 0dog
-
- I’m angry
- 0dog
-
- I recommend it
- dog
Hot news now
It seems that the text you provided discusses recent developments in the field of obesity treatment, particularly focusing on GLP-1 medications like Wegobee and Zebbound. Here’s a brief summary of the key points:
- Effectiveness of Wegobee: Research indicates that Wegobee has shown significant promise in alleviating pain due to its weight loss effects and anti-inflammatory properties. In clinical trials, participants taking Wegobee reported significantly greater reductions in pain compared to those on a placebo.
- Zebbound for Sleep Apnea: In another study, Eli Lilly reported that their GLP-1 treatment, Zebbound (tyrzepatide), effectively reduced the apnea/hypopnea index in patients, leading to improvements in symptoms associated with sleep apnea. They have applied for FDA approval for Zebbound to be used as a treatment for sleep apnea.
- Concerns Over Side Effects: Despite the positive outcomes, there are growing concerns regarding side effects linked to the prolonged use of GLP-1 medications. A recent case highlighted in a study reported a link between increased dosages of semaglutide and severe acute pancreatitis, which ultimately resulted in a patient’s death.
- Potential for Misuse: There is also concern regarding non-obese individuals taking these medications for weight loss, which could lead to misuse. Regulatory bodies, like the Ministry of Food and Drug Safety in South Korea, have issued warnings about the dangers and the need to purchase medications from proper channels to ensure safety and efficacy.
- Awareness on Administration and Dosage: Experts have emphasized that careful attention should be given to dosage and administration of these drugs to minimize the risk of severe side effects, such as pancreatitis.
while GLP-1 medications offer hope for obesity treatment and related conditions, ongoing vigilance is necessary to manage the potential risks associated with their use.

