Controversy over judicial control seen as ‘Invossa innocent’
US FDA, ‘negative regulation’ for new drug development… Once safety is verified, clinical trials will be resumed.
Korea, SK Vassar-Pfizer patent lawsuit… Unlike the European court, sales were banned.
“Competition for new drugs requires the expertise of regulatory authorities”
In the wake of KolonS ‘Invossa incident’, controversy over excessive control by regulatory authorities and investigative agencies in the cutting-edge scientific field is spreading. This is because the difference in response between Korea, wich was marred by litigation, and the United States, which was consistent with scientific verification, was clearly revealed. The court that found Kolon Honorary Chairman Lee Woong-yeol not guilty on the 29th of last month also said, “We need to think deeply about judicial control in the field of science.”
The domestic pharmaceutical and biotechnology industry is raising its voice, saying that for Korea, which has just entered the battle for new drug development, to catch up with advanced countries such as the United States and Europe, it must be supported by regulatory reform and the securing of expertise from the government and judicial authorities.
Seung-gyu Lee, vice president of the korea Biotechnology Association, said, “Not only do regulatory authorities need to have expertise so that they can try new things that have never existed in the world, but ther is also a need to change their perception of new drugs.” He added, “In addition, we need to create an atmosphere where public officials in charge are not disadvantaged in the future even if they make innovative decisions.” “it should also be created,” he said.
● The US FDA uses a ‘negative’ regulatory method… “Do your research first”

The incident with Invossa, the world’s first osteoarthritis gene therapy, began when the ingredient listed by Kolon when it received product approval (sales approval) from the Ministry of Food and Drug Safety in 2017 was confirmed to be a different ingredient two years later. Kolon discovered this during clinical trials in the United States and reported it to the Ministry of Food and Drug Safety and the U.S. Food and Drug Administration (FDA) in 2019.
The responses of the Korean and U.S. authorities diverged here. In Korea, criticism from political circles and civic groups intensified, saying it was ‘purposeful manipulation by a large company’, and
On the other hand, the U.S. FDA put the ongoing phase 3 clinical trial on hold immediately after Kolon’s report and decided to resume the clinical trial in 2020 after reviewing the safety impact. Kolon completed Phase 3 Invossa patient administration in the United States in July of this year.
In the bio industry, the FDA has a ‘negative regulation’ method that allows all activities unless prohibited by law, so even if some procedural problems are found, if there are no problems with the safety and efficacy of a new drug, it has the practice of not blocking research, so clinical trials can be resumed. see
In 2019, Swiss pharmaceutical company Novartis’ gene therapy drug ‘Zolgensma’ was also found to have manipulated some data, but the FDA did not revoke its product approval. He announced that he would separately consider filing complaints or ordering countermeasures only for manipulation. At the time, the FDA emphasized that the manipulated data was part of the manufacturing process and that “it is indeed critically important to promote the development of innovative new drugs, such as cell and gene therapies that can treat untreatable diseases.”
Unlike the United States, Korea uses a ‘positive regulation’ method that prohibits anything except what is permitted. An surroundings where regulatory agencies are passive toward innovation also makes it difficult to develop new drugs. An official from the bio industry said, “Initially, the Ministry of Food and drug Safety tried to foster innovation by quickly granting approval for Invossa, but due to criticism from public opinion, it changed to excessive regulation, and the prosecution also drew its sword,” adding, “The problem is that science is swayed by public opinion.” He said.
● “The new drug patent trial system is also controversial”
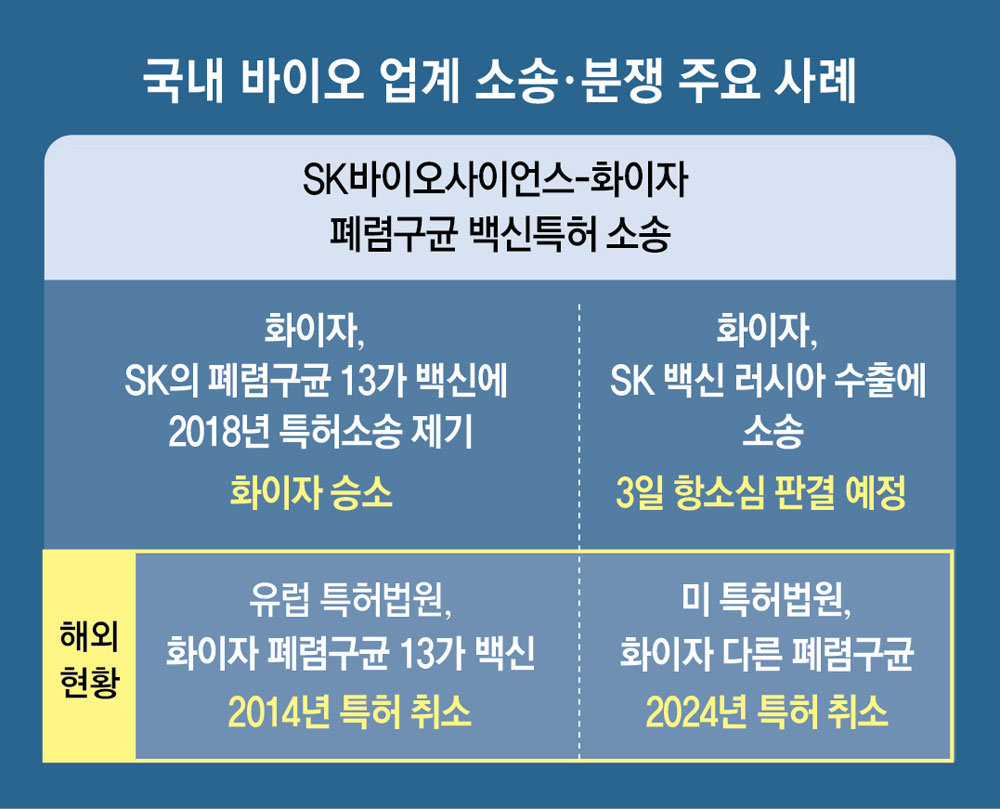
The bio industry agrees that it is important to secure the expertise of the authorities as lawsuits and disputes surrounding Korean new drugs become more frequent and dependence on judicial decisions increases. This is because there are many cases where new drug development, which took more then 10 years, is stranded in lawsuits.
A representative example is the patent lawsuit that has been ongoing in Korea between SK Bioscience and Pfizer for seven years. SK developed Korea’s first pneumococcal 13-valent vaccine and received approval from the Ministry of Food and Drug Safety, but lost a patent infringement lawsuit filed by Pfizer, and domestic production and sales were banned until April 2027. When Pfizer exported the research concentrate to a Russian pharmaceutical company in an attempt to find a sales outlet, Pfizer again filed a lawsuit, and an appeals court ruling is scheduled for the 3rd of this month.
The European Patent Court canceled Pfizer’s patent in 2014 for “lack of originality.” This year, in the united States, a patent invalidation ruling was issued when Sanofi and SK filed a lawsuit against Pfizer’s other pneumococcal vaccine.
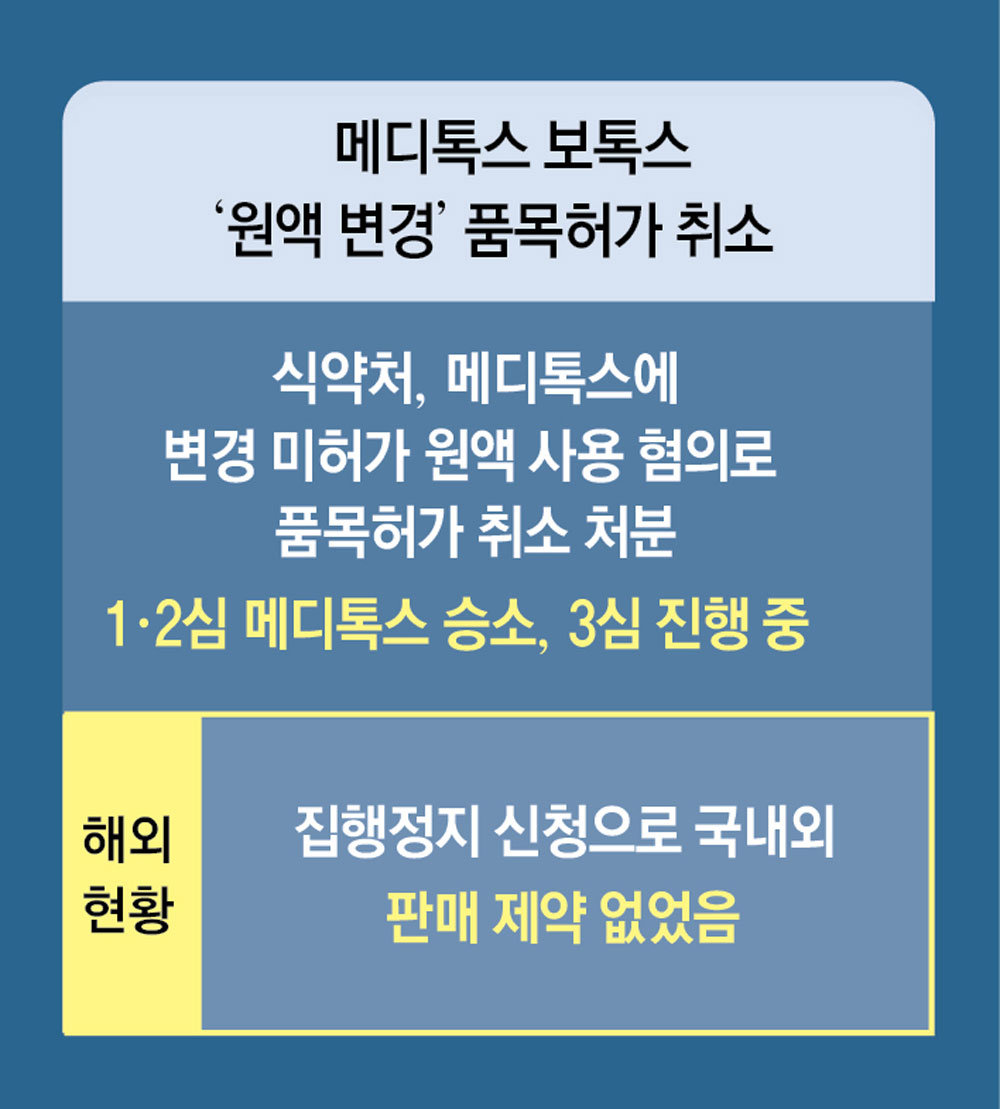
Medyt
The bio industry also argues that the participation of technical experts in patent trials should be institutionally guaranteed, like in advanced new drug development countries such as Europe. This is why a bill was recently proposed in the National Assembly mandating the participation of professional examiners and technical examiners in patent trials. An official in the pharmaceutical industry said,“If a product is not released and is hit with lawsuits,it will be difficult for all but the most global companies to survive,” adding,“The expertise of the Patent trial and Appeal Board and the judiciary will be increasingly required.”
Reporter Nam Hye-jeong [email protected]
Reporter Kim Tae-eon [email protected]
Reporter Jong-ho Han [email protected]
- I’m angry
- 0dog
Hot news now
How can technical expertise be integrated into patent litigation cases for new drug development?
Ers in patent litigation cases involving new drug development. This proposed reform aims to ensure that technical expertise is considered during legal disputes, thereby fostering a more informed and effective judicial process.
The ongoing challenges faced by the Korean pharmaceutical and biotechnology sectors highlight the importance of balancing regulatory oversight with the need for innovation. Industry leaders stress that a supportive regulatory habitat is crucial for Korea to compete effectively on the global stage,especially in the rapidly evolving field of biopharmaceuticals.
As the controversy surrounding Kolon’s Invossa incident continues, stakeholders are pushing for a paradigm shift in how regulatory authorities and judicial systems engage with scientific and technological advancements. This includes fostering a collaborative atmosphere that encourages innovation while maintaining necessary safety and efficacy standards.
the future of the Korean bio industry may heavily depend on regulatory reforms and the integration of expert insights into both regulatory processes and judicial proceedings. As these discussions take center stage, the emphasis remains on creating a legal and regulatory framework that not only protects public health but also nurtures the growth of pioneering therapies that can address unmet medical needs.

