Unraveling the Complexities of MIS-C: New Insights into a Post-COVID Inflammatory syndrome in Children
Table of Contents
The emergence of multisystem inflammatory syndrome in children (MIS-C) following the COVID-19 pandemic has presented a critically important challenge to pediatric healthcare. Recent research is shedding light on the intricate relationship between MIS-C, Kawasaki disease, and the underlying immunological mechanisms driving these conditions, offering potential avenues for improved diagnosis and treatment.
The Overlap Between MIS-C and Kawasaki Disease
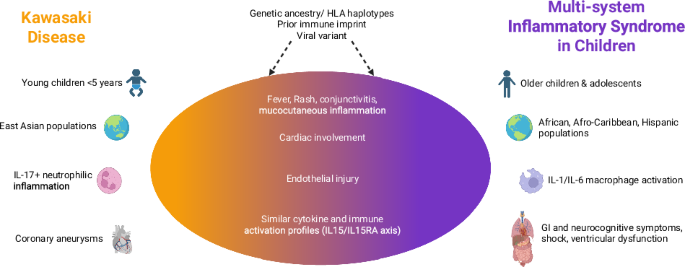
For years, Kawasaki disease (KD) has been a leading cause of acquired heart disease in children. The onset of the COVID-19 pandemic brought a new, similar illness – MIS-C – into focus. Studies, including those by Alkan et al. (2022) and Bar-Meir et al. (2023), have focused on differentiating the two. While distinct, a growing body of evidence suggests a strong connection. One researcher noted that MIS-C often presents with overlapping symptoms, making initial diagnosis tough.Mastrangelo et al.(2025) go further, positing that MIS-C is, in fact, a SARS-CoV-2 triggered form of Kawasaki disease.
Decoding the Immune Response
The immunological landscape of both MIS-C and KD is complex. Research from Consiglio et al. (2020) began to unravel the immunology of MIS-C, revealing a dysregulated immune response in affected children. Further inquiry, such as the work by Vella et al. (2021),demonstrated a marked,though transient,immune activation in MIS-C compared to adult and pediatric COVID-19.
Specifically, studies are pinpointing key players in this inflammatory cascade. Diorio et al. (2021) identified heterogeneity in MIS-C patients relating to interferon gamma dysregulation and vascular endothelial dysfunction. Porritt et al.(2021) highlighted an autoimmune signature in the hyperinflammatory syndrome, while Brodeur et al. (2022) found elevated IL-17 cytokines distinguish Kawasaki disease from other pediatric inflammatory disorders. Pro tip: elevated IL-17 cytokines can be a key differentiator in diagnosing Kawasaki disease. An artificial intelligence-guided signature, as revealed by Ghosh et al. (2022),even points to a shared host immune response in both MIS-C and KD. Beltran et al. (2023) added another layer of understanding, revealing shared immunological drivers through single-cell meta-analysis of neutrophil activation.
The role of genetics in susceptibility to MIS-C is also under scrutiny. Chou et al.(2022) investigated the mechanisms underlying genetic susceptibility, suggesting a complex interplay between viral exposure and individual genetic factors. Sancho-Shimizu et al. (2020) propose that SARS-CoV-2-related MIS-C could hold the key to understanding the viral and genetic causes of Kawasaki disease itself.
Shifting Risk with Emerging Variants
The risk of developing MIS-C appears to have evolved alongside the SARS-CoV-2 virus. Cohen et al. (2022) reported a lower risk of MIS-C in children infected with the delta and Omicron variants, suggesting viral evolution may influence the severity and incidence of the condition.
Global Surveillance and Understanding
The impact of MIS-C is not limited to a single region. Matsubara et al. (2021) conducted a nationwide survey in Japan, providing valuable data on the prevalence and characteristics of MIS-C in a different population. Sacco et al. (2021) further contributed to the understanding of immunopathological signatures in both MIS-C and pediatric COVID-19.
The ongoing research into MIS-C is crucial for refining diagnostic criteria,developing targeted therapies,and ultimately improving outcomes for children affected by this complex post-COVID inflammatory syndrome. Continued investigation into the shared immunological pathways with Kawasaki disease may unlock new strategies for prevention and treatment of both conditions. Reader question: How can parents identify early symptoms of MIS-C in their children?