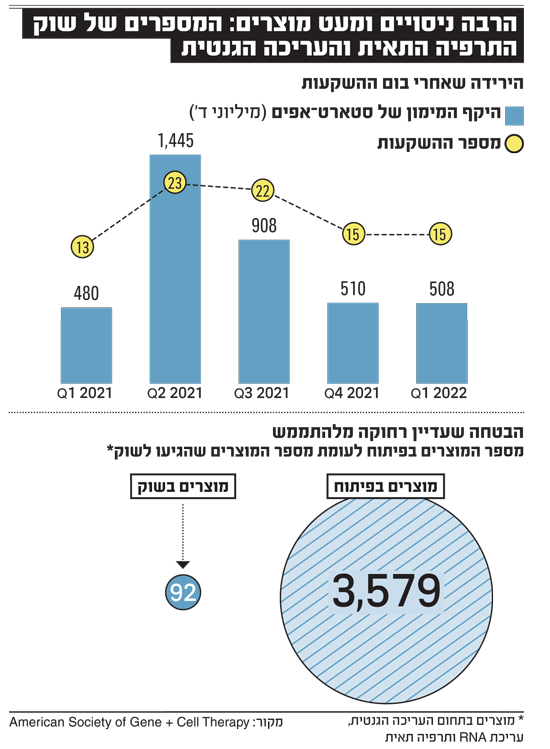
It is rare for one product to make two impressive scientific breakthroughs at once, but CAR technology-T, cell-mediated cell therapy, did just that. The technology, which is known in Israel mainly thanks to Kite Pharma, the leading companyLuca, founded by Prof. Arie Baldgreen on the basis of Prof. Zelig Eshchar’s invention, made it possible to remove immune system cells from a patient, modify them genetically, and return them to the body, which are specially strengthened and aimed at eliminating leukemia. This treatment was concurrent with the first commercial treatment using live cells (other than bone marrow transplantation) and the first commercial treatment performed by genetic engineering of the patient’s own cells.
Since then, cell therapy and genetic engineering have become a hot commodity. Combined with the stock market tide, the pricing of companies operating in these areas has skyrocketed. These days have passed, or are passing in these very moments, and the market is becoming more stingy with the cash it is willing to distribute to technology companies in general and biomed in particular. Companies with spectacular science but far from the market have suffered a painful drop in their fundraising capacity, but are still rich in cash from previous fundraisers and are preparing for scientific breakthroughs.
At the recent Biomed 2022 conference in Tel Aviv, we spoke with three executives from young and intriguing companies in these fields, who are rushing forward to get proof quickly, before it is time to recruit again. The three are Zen Lee, founder and CEO of ADARx Pharmaceuticals, which operates in the field of genetic editing using RNA; Adele Nada, CEO of Gentibio, which develops immune cells for the treatment of inflammatory diseases; And Chen Schur, CEO of Addist Bio, an Israeli-American company that develops cells that are supposed to be the next generation of CAR-T.
From euphoria to hangover: what happened to CAR-T
Do you also feel that there is a decrease in the euphoria that the fields of cellular and genetic therapy have created in recent years?
Nada: “There is, so to speak, a ‘hangover’ around the CAR-T field. It started with euphoria: CAR-T cells showed unprecedented results in some types of leukemia, even curing in some cases. The hangover was due to commercial reasons. Pharma companies entering this field encountered commercialization challenges due to the cost of the product and the supply chain, especially during the corona period (treatment requires collecting cells from the patient, flying them to the center where the genetic engineering is performed and returning them to a patient who is cancerous and not always mobile). – G.).
“But in my opinion, if we do succeed in reaching a product that cures disease by a good percentage, then all these problems are secondary. We believe that we can really, as already happens in CAR-T but rarely – reach a situation where after our treatment no further treatment will be needed. “It already makes the whole story lucrative for healthcare systems and pharma companies, even with existing supply chain issues.”
Taurus: “The commercial and logistical challenges in CAR-T products mean that only 20% of those who could have actually received them, and that is in developed countries. In addition, 70% of tumors return to progress after six months.”
We know why tumors stop responding to existing treatment?
Nada: “In some cases the cancer cells stop expressing the receptor to which the CAR-T cell is supposed to attach. In other cases, it happens for reasons that are not fully understood, maybe there are not enough T cells or they are not strong enough.”
Taurus points to another problem, related to the safety of care. Today, it is used in alpha-beta cells (as opposed to gamma-delta cells used by Addist), and he says they have built-in safety issues. “They are associated with the release of cytokines (small proteins secreted mainly by immune system cells, and their excessive secretion can be harmful – CW). It can be managed through immunosuppression, but it is complicated. “
Chen Shor / Photo: PR
To deal with these difficulties, Nada says, Gentibio is also developing a product sourced from the patient himself, whose production process is relatively complex but his time for marketing approval is shorter, as well as a shelf product, which will be produced from healthy volunteer cells (allogeneic cells). It is supposed to solve some of the logistical problems.
“If the product waits for the patient at the treatment center, the costs and logistical requirements will be significantly reduced, and then the number of patients receiving the treatment will also increase,” he says. “We believe we have been able to solve the safety problem in the allogeneic cells, so the main problem is that a good source of these cells needs to be identified and it must be very cheap.”
In addition to solving accessibility and safety issues for the patient, the cells that will not be eliminated by the immune system should also be protected in order for the treatment to be effective, Nada says. “The immune system can be suppressed, but then we can impair positive processes in the body. So we genetically edit our cells so they have a better chance of surviving. We only perform one edit. There are other approaches, based on several edits, which is already more dangerous, Because any editing gives the cell features whose full effect can only be understood experimentally. “
Editing with RNA: The goal is to reach the depths of the brain
Going back to the challenges of genetic editing, how are they expressed in companies like ADARx, which perform the editing within the body using RNA?
Lee: “The main problem is the conduction into the cell. We use the receptors on the cell and a component on our drug that binds to these receptors and forces them to insert the RNA into the cell. In the meantime, we found this process safe. Because we know exactly what RNA sequence we are using In it, we can expect his behavior. “

Zen Lee, Founder and CEO of AdarX / Photo: PR
One of the benefits of editing within the body, mine points to, is the cost. “The amount of material required for a patient is very small – grams every year – so there is no real cost of material. This is an advantage over cells held outside the body. Because the cost of the material and our logistical cost are so low, we get a much larger share of insurance coverage. Part of the induction goes to the process of producing the cells, transporting them, processing them and returning them – c). If we talk in the future about common neurological diseases, like Alzheimer’s, we are talking about a lot of patients who need treatment and are very difficult to treat. “Compared to the options that are being talked about today of frequent transfusions, the possibility of a semi-annual injection or even at a larger interval is amazing.”
One who has broken through in this field is alnylam, which has developed drugs that manipulate RNA within the body.
Lee: “It’s a right-wing marker for the field, and its leading product has been acquired by Novartis. Its other products are aimed at rare diseases and the company is succeeding with them not bad at all (the company’s market value today stands at about $ 15 billion). We target diseases that are even more difficult to treat, with products that need to be much more potent and selective. Our uniqueness is in reaching the depths of the mind. To date, no one has done this and we have the option to be there first. “If we add to the treatment of rare diseases both the treatment of common diseases and the treatment of neurological diseases, we are already talking about markets of billions of dollars for any future RNA drug, and there will be dozens of such.”
Do we understand the brain diseases enough to know what to do there, when we get there?
Lee: “Oh, that’s a great question. We know how to reach the brain, and we need goals in the brain that have been validated, which means they tell us where to act to really achieve a result and improve the disease. Today there are not enough well-defined goals in the brain. “
Missing study: Mapping the genetic trajectory of diseases
When panelists are asked about how they see the future of genetic editing, they point in several directions. Lee talks about RNA editing, which makes a specific and reversible change, alongside DNA editing, which makes a permanent change. “The choice between them will depend on the person and the illness, how much they want the change to be permanent versus safe. It will depend on the test of the result, and the sentiment of the regulator.”
According to her, her company is already working on genetic editing technology, alongside RNA technology. “It’s still in the research phase, but we’m definitely talking about the ability to cancel a gene or change a gene regularly. It would probably be right to make both types of changes only in a particular tissue so that repair can be made where a particular gene does damage without affecting where it may be needed. “For example, cells that stop responding well in the brain and cause Parkinson’s, maybe we can give them some boost without disturbing all the other cells in the body.”
Taurus says that editing done in the past outside the body can also be done inside it. “There is evidence that CAR-T cells can also be edited within the body,” he said.
Nada adds that genetic editing will become increasingly specific. “In the past, it was not very specific, but it has become so thanks to CRISPR technology. Not every cell therapy technology has to be specific at this level, but there is no doubt that things can be done that could not be done in the past.”
How is the information revolution and artificial intelligence changing the field?
Taurus: “In the future we see artificial intelligence as an aid in choosing the goals in the body to which we will direct our cells.”

Adele Nada, CEO of Gentibio / Photo: PR
Nada: “Computerized design of the physical structure of drugs is very relevant in drugs based on small chemical sequences and proteins, and less relevant to RNA and cell therapy. The cell knows how to reach a specific tissue and do something specific, and not have to computerize it. We have problems. Larger solutions to solve first, such as supply chain management. “
Lee: “We do use bioinformatics, especially mapping the genome and understanding the relationship between genetics and clinical outcomes, to choose our goals. In the future, I see artificial intelligence being used in the field of personalized medicine. Is different because his genetics are a little different, so we’ll tailor the product for him. It’s completely on the horizon, and that’s another benefit to our technology. Is there an ‘Alzheimer’s gene’ or does every person with Alzheimer’s express a slightly different problem gene? The way to attack it. “
What do you need from academia or industry?
Nada: “We want to better understand the immune system. We want to better understand why the immune system goes wrong and what Treg cells do exactly to restore its balance, and how it can be done better. These are insights that are really needed from academia.”
Lee: “We believe that all diseases in the world have some genetic origin, and we would like to see a mapping of the genetic pathways of the various diseases and how they are expressed in the disease, so that we can address them through our drugs.”
AdarX
Field of Activity: Genetic editing using RNA. Develops products for hereditary emphysema, rare diseases due to deficiency or excess protein, heart disease and brain disease. If the RNA can be modified to produce the missing protein successfully, the existing protein infusions can be replaced with a low-dose and less frequent injection.
history: An American company founded in 2019
data: Recently completed a $ 75 million fundraiser
Genibio
Field of Activity: Develops Treg cells (Regulatory T cell) for the treatment of inflammatory diseases. Treg cells are the independent control system of the immune system and their purpose is to regulate its activity. The company uses technology from the Israel Miguel Institute to engineer T cells to become Treg cells with targeting capabilities in certain tissues. The goal is no less than to cure inflammatory diseases and even diabetes. Meanwhile, the company has shown a significant regression of juvenile diabetes in mice.
history: An American company established in 2020
data: Raised $ 157 million last August. Among the investors was Novartis, Big Pharma’s most active in the field of cellular therapy
Will add Bio
Field of Activity: Develops cells that are supposed to be the next generation of CAR-T. The company produces alpha-beta cells, which are common today, gamma-delta cells that do not stimulate the patient’s immune system.
history: It was established in 2014 in Israel on the basis of Israeli technology and today operates from the United States. Since 2018, it has been traded on NASDAQ.
data: Raised $ 80 million in 2019. Today it is traded on the NASDAQ at a value of $ 460 million
