2023-05-08 15:56:19
WAKIX® recently received the approval extension for children and adolescents from 6 years. This sensitive group can therefore also benefit from an innovative and effective therapy with a favorable safety profile and simple application.1
Narcolepsy usually begins in childhood
Narcolepsy is a chronic neurological disease. It is characterized by an instability of the sleep-wake cycle and excessive daytime sleepiness without (narcolepsy type 2) or with cataplexy (narcolepsy type 1). In the majority of cases, the first symptoms already appear in children and adolescents, with a peak at the age of about 15 years. A second, smaller peak is observed in the mid-30s of life.2,3
Narcolepsy is often only correctly diagnosed after many years
Pediatric patients present a particular challenge for diagnosis because their narcolepsy symptoms may present differently than adults and may also change over time. Children are often less able to describe their condition and sometimes react paradoxically to sleepiness, for example with irritability and hyperactivity. It can therefore easily lead to misinterpretations and incorrect diagnoses.3-5
The suffering of the children is very high. Narcolepsy can lead to school problems, mental health problems, and social withdrawal, especially when it is unrecognized and a plausible explanation for the symptoms is lacking. An early and correct diagnosis with appropriate treatment is therefore essential for the best possible development of these children. 3-5
WAKIX® provides wakefulness with a unique mechanism of action
Pitolisant is a selective histamine H3 receptor antagonist/inverse agonist. The H3 receptor is an autoreceptor that controls histamine concentration presynaptically in the histamine neurons in the brain and inhibits both its release and the endogenous production of histamine. Pitolisant blocks the histamine H3 autoreceptor so that the histamine, which promotes wakefulness, can be produced and released in excess. Vigilance, attention, performance and memory improve in this way. WAKIX® thus has a positive effect on narcolepsy symptoms, both through direct effects on the histaminergic system and through indirect effects on other neurotransmitters that promote wakefulness, such as norepinephrine, dopamine and acetylcholine.1,6
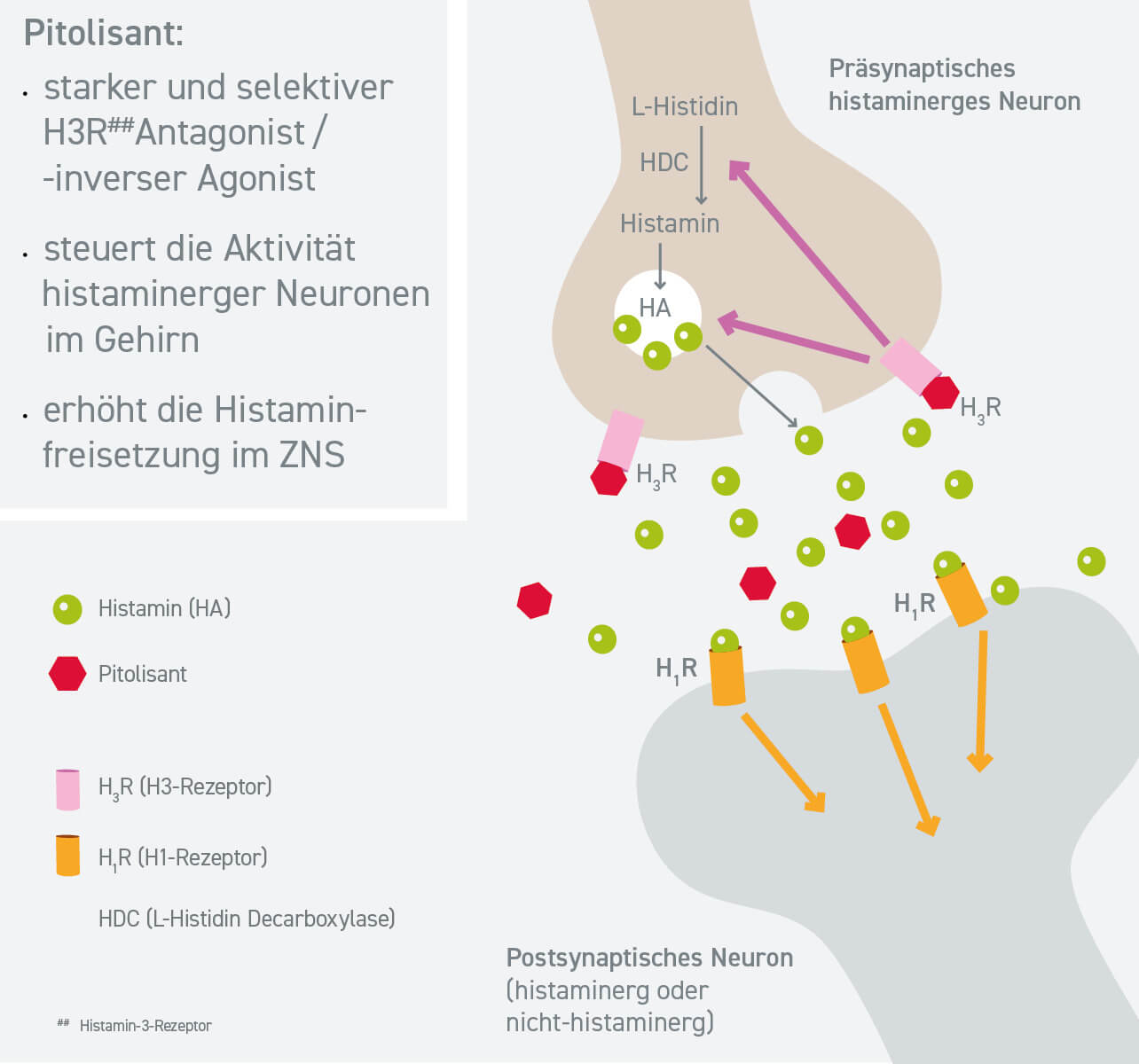 Mod. nach: Benarroch EE. Neurology. 2010; 75 (16):1472-1479.
Mod. nach: Benarroch EE. Neurology. 2010; 75 (16):1472-1479.
WAKIX® – a simple and effective therapy for all forms of narcolepsy
WAKIX® was approved in Europe in 2016 for the treatment of narcolepsy in adults. Recently, the approval was extended to the treatment of children from 6 years and adolescents with narcolepsy with and without cataplexy.1 This is a major advance because among the very limited treatment options approved for children with narcolepsy, WAKIX® offers the advantage of being broadly and scientifically proven to treat both daytime sleepiness and cataplexy. It is beyond that not considered Classified as a narcotic and non-psychostimulant. Treatment with WAKIX® film-coated tablets is very simple. It is sufficient to take the daily dose once a day in the morning, preferably during breakfast. The daily dose should be titrated up, in children starting at 4.5 mg and doubling the dose weekly. A dose of 36 mg/day should not be exceeded. In children under 40 kg, the maximum daily dose is 18 mg.
Classified as a narcotic and non-psychostimulant. Treatment with WAKIX® film-coated tablets is very simple. It is sufficient to take the daily dose once a day in the morning, preferably during breakfast. The daily dose should be titrated up, in children starting at 4.5 mg and doubling the dose weekly. A dose of 36 mg/day should not be exceeded. In children under 40 kg, the maximum daily dose is 18 mg.
WAKIX® significantly improves both daytime sleepiness and cataplexy in children
The efficacy and tolerability of WAKIX® examined. The primary endpoints were excessive daytime sleepiness and the number of cataplexy attacks, which were summarized in a total score in the Ullanlinna Narcolepsy Scale (UNS). WAKIX® reduced the combined endpoint of daytime sleepiness and cataplexy in the UNS total score very significantly and clinically relevant compared to placebo.9
In the individual parameters (UNS cataplexy partial score and Pediatric Daytime Sleepiness Scale (PDSS)), WAKIX® was better in the placebo group both in reducing cataplexy attacks (p=0.023) and in reducing daytime sleepiness (p=0.002). ) significantly superior.1,7
WAKIX® also has a favorable safety profile in children
The generally good tolerability of pitolisant, which is known from the studies in adults, was confirmed in the children’s study. Frequency, type and severity of adverse reactions were mostly mild in nature and there were no severe or serious treatment-emergent adverse reactions. The most common were headache (11%), insomnia (5.5%) and hypertension (2.7%).1
long-term data over 1 year are available in an open study for adults and showed that the clinical effect of pitolisant improved continuously over 12 months. After 1 year, 2/3 of the patients could be classified as responders and 1/3 as normalized with regard to daytime sleepiness. Cataplexy attacks were reduced by 68%, hallucinations, sleep paralysis, and sleep attacks by 54%, 63%, and 27%, respectively. The safety profile also remained unchanged compared to shorter studies. The frequency of adverse events decreased after 3 months of therapy.8
Overall, the data for WAKIX® has a very good risk-benefit profile and the approval for children underscores this good profile of the active substance pitolisant.
credentials
- Information for professionals Wakix® (Bioprojet Pharma), as of March 2023.
- Bassetti CLA et al. Narcolepsy-clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol 2019;15:519–539.
- Maski K et al. Listening to the patient voice in narcolepsy: Diagnostic delay, disease burden, and treatment efficacy. J Clin Sleep Med 2017;13:419–425.
- Plazzi G et al. Clinical characteristics and burden of illness in pediatric patients with narcolepsy. Pediatr Neurol 2018;85:21–32.
- Chung I-H et al. Pediatric narcolepsy – a practical review. Children 2022;9:974.
- Guevarra JT et al. Pitolisant to treat excessive daytime sleepiness and cataplexy in adults with narcolepsy: Rationale and clinical utility. Nature Sci Sleep 2020;12:709–19.
- Dauvilliers Y et al. Safety and efficacy of pitolisant in children aged 6 years or older with narcolepsy with and without cataplexy: a double-blind, randomized, placebo-controlled trial. Lancet 2023;22:P303–11.
- Dauvilliers Y et al. Long-term use of pitolisant to treat patients with narcolepsy: Harmony III study. Sleep 2019;42:1–11.
Wakix® 4.5 mg/ -18 mg film-coated tablets. Active substance: pitiful. Composition: Each film-coated tablet contains pitolisant hydrochloride equivalent to 4.45 mg/-17.8 mg pitolisant. Other ingredients: Microcrystalline cellulose, crospovidone type A, talc, magnesium stearate (Ph.Eur.), colloidal anhydrous silica, poly(vinyl alcohol), titanium dioxide (E171), macrogol 3350. Application areas: Wakix is used in adults, adolescents and children from 6 years of age to treat narcolepsy with or without cataplexy. Contraindications: Hypersensitivity to the active substance or to any of the excipients, severe hepatic impairment, lactation. Side effects: Frequently: Insomnia, anxiety, irritability, depression, sleep disorder, headache, dizziness, tremor, vertigo, nausea, vomiting, dyspepsia, fatigue. Occasionally: Decreased appetite, increased appetite, fluid retention, agitation, visual/audible hallucination, affect lability, abnormal dreams, dyssomnia, difficulty staying asleep, difficulty falling asleep, premature awakening, nervousness, tension, apathy, nightmares, restlessness, panic attack, decreased libido, increased libido, suicidal thoughts, dyskinesia , balance disorder, cataplexy, disturbance in attention, dystonia, on-off phenomenon, hypersomnia, migraine, psychomotor hyperactivity, restless legs syndrome, somnolence, epilepsy, bradykinesia, paraesthesia, decreased visual acuity, blepharospasm, tinnitus, extrasystoles, bradycardia, hypertension, hypotension, Flushing, yawning, dry mouth, abdominal pain, diarrhoea, abdominal discomfort, abdominal pain upper, constipation, gastroesophageal reflux disease, gastritis, gastrointestinal pain, hyperacidity, oral paresthesia, gastric discomfort, erythema, pruritus, rash, hyperhidrosis, sweating, arthralgia, back pain, muscle rigidity, muscle weakness, Musculoskeletal pain, myalgia, pain in extremity, pollakiuria, metrorrhagia, asthenia,
Chest pain, feeling abnormal, malaise, edema, peripheral edema, weight increased, weight decreased, liver enzymes increased, ECG: prolonged QT interval, increased heart rate, increased gamma-glutamyltransferase. Rarely: Anorexia, hyperphagia, appetite disorder, behavior abnormal, confusional state, depressed mood, excitability, obsessive thoughts, dysphoria, hypnopompic hallucination, depressive symptom, hypnagogic hallucination, mental impairment, loss of consciousness, tension headache, memory impairment, poor sleep quality, abdominal distension, dysphagia, flatulence, painful swallowing, enterocolitis, toxic rash, photosensitivity, neck pain, musculoskeletal chest pain, spontaneous abortion, pain, night sweats, anxiety, creatine phosphokinase increased, general condition abnormal, ECG: repolarization irregularity, ECG: T-wave reversal. Warnings: Store drug out of reach of children. sales accrual: Prescription only. Authorization holder: Bioprojet Pharma, 9, rue Rameau, 75002 Paris, France. Status of information: 03/2023.
 This medicine is subject to additional monitoring. This enables rapid identification of new security insights. Healthcare professionals are asked to report any suspected adverse reactions. For information on how to report side effects, see section 4.8 of the prescribing information.
This medicine is subject to additional monitoring. This enables rapid identification of new security insights. Healthcare professionals are asked to report any suspected adverse reactions. For information on how to report side effects, see section 4.8 of the prescribing information.
#WAKIX #pitolisant #children #narcolepsy #cataplexy
