Researchers at North Carolina State University and the University of North Carolina at Chapel Hill have developed a biotechnological implant that produces and releases CAR-T cells to attack cancer. In a proof-of-concept study conducted on a mouse model of lymphoma, published in Nature Communications, researchers found that treatment with the implants was faster and more effective than conventional treatment against CAR-T cell cancer.
T cells are part of the immune system; they are responsible for identifying and destroying cells that have been infected with an invading pathogen. CAR-T cells are T cells designed to
identify cancer cells and destroy them and are already being used in the clinic to treat lymphomas, and there are many ongoing clinical trials focusing on the use of CAR-T cell treatments against other types of cancer.
“A major drawback of CAR-T cell treatment is its cost,” acknowledges Yevgeny Brudno, author of the study.
For this reason, Brudno points out, “many people are excluded from this treatment. One of the reasons for the high cost is that the manufacturing process is complex, time-consuming and must be tailored to each cancer patient individually.”
“Reducing manufacturing time is very important for patients with rapidly progressing diseases,” adds Pritha Agarwalla.
To resolve this situation, researchers have innovatively designed biotechnological implant which they have named ‘Multifunctional Alginate Scaffold for T-Cell Engineering and Release’ (MASTER).
To understand how MASTER works, you must first understand how CAR-T cells are produced.
It is a complex process. In a first phase, T cells from patients are isolated and transported to a clean manufacturing facility. At the facility, the researchers “activate” the T cells with antibodies for several days, preparing them for reprogramming.
Once the T cells have been activated, viruses are used to introduce the CAR gene, reprogramming the T cells into CAR-T cells that target cancer cells. Factors are then added to stimulate the proliferation of CAR-T cells, thus expanding their number. Finally, once these manipulations are complete, a process that can take weeks, the cells are returned to the hospital and infused into the patient’s bloodstream.
“Our MASTER technology “skips” cumbersome and time-consuming activation, reprogramming, and expansion steps because they take place inside the patient Agarwalla explains. This reduces a process from several weeks to a single day.”
With this innovative treatment, researchers isolate the patient’s T cells and mix them with the engineered virus. Next, this mixture is poured over the MASTER, which absorbs it.
MASTER contains the antibodies that activate T cells, so the cell activation process begins almost immediately. Meanwhile, MASTER is surgically implanted in the patient; in these studies, a mouse.
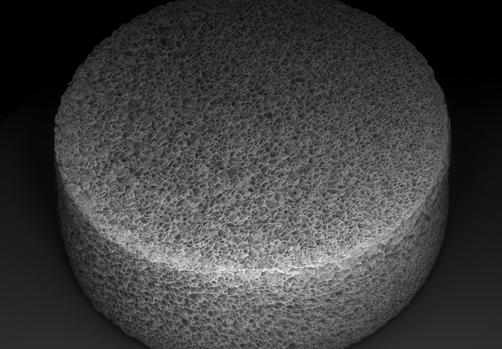
After implantation, the process of cell activation continues. As the T cells become activated, they begin to respond to the modified viruses, which reprogram them into CAR-T cells.
MASTER is made of biocompatible, sponge-like material with the look and feel of a mini marshmallow. “The large pores and spongy nature of the MASTER material brings the virus and cells closer together, facilitating cellular genetic reprogramming,” explains Agarwalla.
The material is also impregnated with factors called interleukins that promote cell proliferation. After implantation, these interleukins begin to leak, promoting rapid proliferation of CAR-T cells.
In their studies, the researchers worked with a mouse model of lymphoma.
One group was treated with CAR-T cells that were manufactured and administered using MASTER. A second group was treated with CAR-T cells that were designed in a conventional way and administered intravenously. These two groups were compared to the control group that received unmodified T cells.
“Our technology worked very well,” admits Brudno. While it would take at least two weeks to make CAR-T cells from naive T cells for clinical use, we were able to introduce MASTER into a mouse within hours of isolating naive T cells.”
In addition, since cells are implanted within hours of isolation, minimal manipulation results in healthier cells that exhibit fewer markers associated with poor anticancer performance in CAR-T cells.
more efficient
In other words, they explain in their work, MASTER generates less differentiated cells, which translates into a better permanence in the body and more anti-cancer power. In addition, the cells show fewer markers of T-cell depletion, which is defined by poor T-cell function.
“The end result is that the mice that received the CAR-T cell treatment through MASTER responded much better to tumors than the mice that received the conventional CAR-T cell treatment,” Agarwalla says.
The improvement in anticancer efficacy was especially pronounced in the long term, when the mice faced a recurrence of lymphoma.
Scientists are now working with this technology on solid tumors, such as pancreatic cancer and brain tumors.
