Stock
nature
-4.17%
Base:3,283
opening:3,196
High:3,244
low:3,141
change:38,145,564
Page Quote News Graphs Company Profile Recommendations
More articles on the subject:
Prominent in the 2.2% decline against the background of the FDA’s (Food and Drug Administration) rejection of a drug for schizophrenia approval. This is not yet the end of the verse, Teva and its partner MedinCell may try to change the outline of clinical trials to meet targets, but at best it greatly delays the trial and at worst completely stops the development of the drug.
Teva’s management, which previously presented the drug as one of the company’s flagship drugs, said in a report on the FDA letter that Teva and its partner remain committed to developing the drug for schizophrenia and providing patients with the product as soon as possible. Teva and its partner, the company’s executives said, are examining the next steps based on the letter and they will work closely with the FDA and in accordance with its recommendations.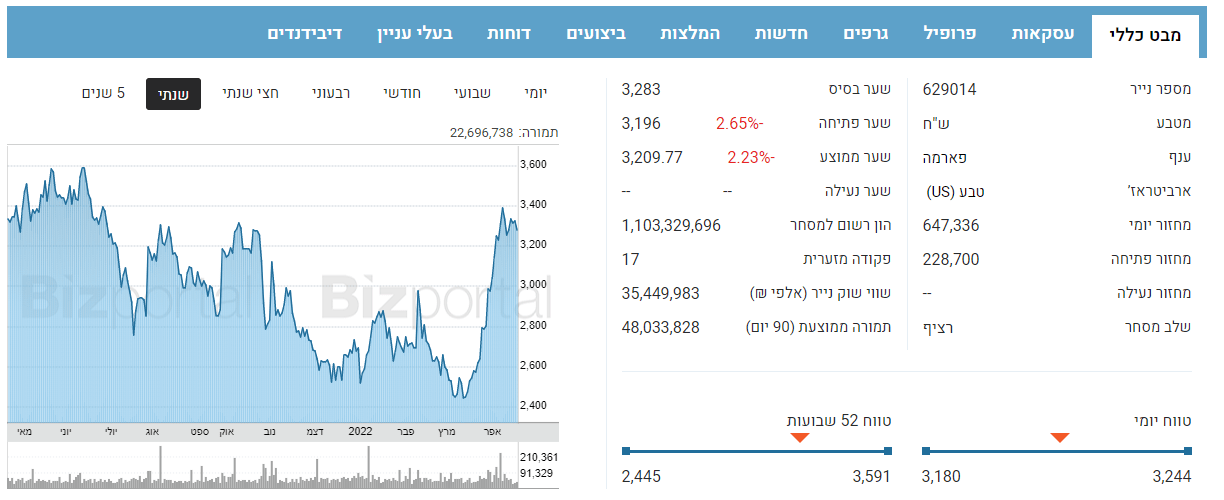
MeninCell CEO Christoph Duet said, “We fully trust our Teva partner to fix what is needed quickly, given the positive results presented in the Phase III clinical trial of the drug. Teva continues to be confident in MedinCell’s technology. ”
Teva is trying in every way to overcome the decline in Copaxone sales that has been going on for 4 years, with Copaxone only recently becoming the second drug in the company’s original product basket (expansion here). The drug for the treatment of schizophrenia was supposed to be one of the future growth engines and probably against this background, the disappointment in the market. Teva CEO Carr Schultz recently estimated that Teva will be able to get approval to market the drug in the U.S. during the first half of the current year – this as stated will not happen.
Data released by the company in the past has shown that it is clinically effective and its range of effect is fast – it begins to affect patients within 24 hours of receiving the first injection. Teva then noted that the drug can be obtained on a monthly or bi-monthly basis. Schultz linked this drug alongside Teva’s powerful drugs when he said recently – “We anticipate that our flagship products Ostedo and Ajobi will continue to grow, and that we will continue to promote our core business through the launch of quality generic drugs worldwide. We are also excited to receive The expected FDA approval for Risperidone LAI, which is an important and significant treatment for patients suffering from schizophrenia. ”
One way or another, investors in nature are primarily interested in developments in connection with opioid compromises. Teva has been sued by US states for three marketing and distribution of addictive painkillers. Teva is not alone, but it is considered one of the most prominent drug distributors. In recent months there seems to be a positive direction This is Teva’s way of rolling out the troubles for years to come and also succeeding in surviving the near future and the future – once the thaw is spread over decades it can be met and also – its significance for the present will be low due to the fact that Teva pays In its cheapest currency – medicines (the cost is very low in relation to the sale price) and also because this future expenditure must be capitalized over decades.
Comments on the article(0):
Your response has been received and will be published subject to system policies.
Thanks.
For a new response
Your response was not sent due to a communication problem, please try again.
Return to comment
