Revolutionary Microscopy Technique Offers Unprecedented View of Engineered Heart Tissue
Table of Contents
- Revolutionary Microscopy Technique Offers Unprecedented View of Engineered Heart Tissue
- The Challenge of Cardiac Tissue Characterization
- How Mid-Infrared Photoacoustic Microscopy Works
- Benefits of a Label-Free Approach
- Demonstrating the Technology’s Potential
- Implications for Regenerative Medicine and Beyond
- Streamlining Tissue Engineering Workflows
- Future Directions and Expanding Applications
A new era in cardiac tissue engineering is dawning with the development of label-free mid-infrared dichroism-sensitive photoacoustic microscopy, a technique poised to dramatically improve our understanding of heart tissue structure and function. This groundbreaking innovation promises to accelerate the development of regenerative therapies for cardiovascular disease, a leading cause of morbidity and mortality worldwide.
The Challenge of Cardiac Tissue Characterization
Histological analysis has long been a cornerstone of evaluating the architecture of bioengineered cardiac constructs. However, traditional methods often rely on invasive staining procedures that can compromise tissue integrity and lack the sensitivity to reveal crucial molecular-level details. Researchers have sought a method to overcome these limitations, and this new microscopy technique appears to deliver.
How Mid-Infrared Photoacoustic Microscopy Works
The newly developed technique combines the strengths of two powerful imaging modalities: mid-infrared light and photoacoustic microscopy. Mid-infrared light excels at probing molecular vibrations, while photoacoustic microscopy converts absorbed optical energy into detectable acoustic signals. Crucially, the addition of dichroism sensitivity allows for the detection of anisotropic molecular orientations – the alignment of molecules within the tissue.
As one researcher explained, the principle hinges on the unique way molecules absorb light. “Mid-infrared light excites vibrational modes of molecules like proteins and lipids, generating acoustic waves that reveal detailed structural alignment within the tissue,” they stated. By exploiting this phenomenon, the system unveils intricate histological features without the need for potentially disruptive dyes or markers.
Benefits of a Label-Free Approach
This label-free imaging technique offers several key advantages. It preserves the native state of the tissue, enabling high-resolution, chemically specific imaging. This is particularly important for engineered heart tissues, where the precise alignment of cellular and extracellular matrix components dictates functionality. The ability to monitor tissue maturation in real-time, without altering its natural state, is a significant leap forward.
Furthermore, the use of mid-infrared wavelengths addresses a long-standing challenge in biomedical imaging: balancing penetration depth with molecular specificity. Unlike shorter wavelengths that lack chemical contrast, or longer wavelengths with limited penetration, the photoacoustic effect allows for deep tissue interrogation without sacrificing molecular detail.
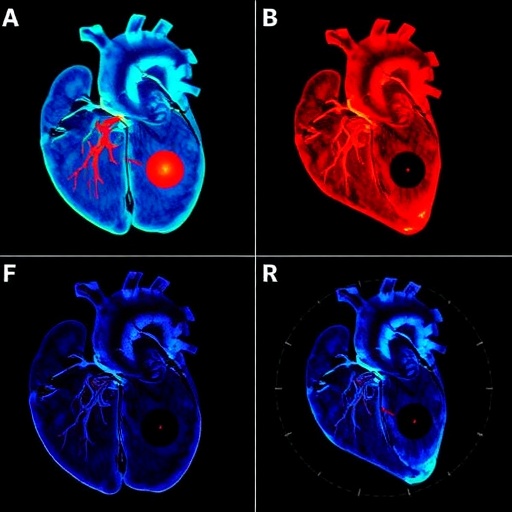
Demonstrating the Technology’s Potential
The research team successfully demonstrated the method on various bioengineered cardiac constructs, visualizing key features such as fiber alignment, cell distribution, and extracellular matrix composition. Compared to conventional imaging, the mid-infrared photoacoustic approach provides superior chemical specificity and spatial resolution, facilitating a direct correlation between structural features and functional properties.
. A visual comparison of traditional staining methods versus the new photoacoustic microscopy would be highly illustrative.
Implications for Regenerative Medicine and Beyond
The compatibility of this platform with live tissue environments opens exciting possibilities for longitudinal studies of tissue development and disease progression. This dynamic monitoring capability is transformative for regenerative medicine, where the functionality of bioengineered tissues must be rigorously validated before clinical application.
Beyond structural imaging, the detailed spectroscopic information provided by the system enhances its diagnostic potential. By identifying specific molecular fingerprints, the technique could detect pathological changes or deviations in tissue composition, paving the way for personalized medicine approaches.
Streamlining Tissue Engineering Workflows
Integrating this photoacoustic microscopy paradigm into existing cardiac tissue engineering workflows promises to streamline the validation process. Researchers and clinicians can benefit from expedited, non-destructive assessments that preserve valuable samples for further analysis. This fosters a more efficient pipeline from laboratory development to clinical translation.
The convergence of optical physics, acoustics, and bioengineering embodied by this technique showcases the power of interdisciplinary collaboration. As the technology matures, scaling and automation will likely facilitate its widespread adoption in routine tissue analysis laboratories and regenerative medicine clinics.
Future Directions and Expanding Applications
Looking ahead, researchers envision expanding this approach to other tissue types where molecular orientation and composition are critical, such as neural, musculoskeletal, and connective tissues. Coupling the technique with machine learning algorithms could further accelerate data interpretation and enable rapid phenotyping and quality control of engineered tissues at scale.
The implications of this innovation extend beyond engineered heart tissues. The foundational principles could inspire a new generation of label-free imaging techniques capable of capturing the complexities of tissue biology with minimal preparation and maximal informational content. This shift toward non-invasive, chemically informative imaging heralds a new era in histopathology and tissue engineering research.
In summary, the advent of label-free mid-infrared dichroism-sensitive photoacoustic microscopy represents a significant leap forward in biomedical imaging and tissue engineering. Its unique ability to combine chemical specificity, structural resolution, and deep tissue penetration without exogenous labels positions it as an indispensable tool for the future of cardiac tissue research and therapy development.
As this technique gains wider acceptance and undergoes further refinement, it is poised to become a cornerstone method for the histostructural analysis of engineered tissues, ultimately contributing to improved clinical outcomes for patients suffering from heart disease and potentially a broad spectrum of other disorders where tissue architecture is a critical parameter.