Worried..it’s true!! The Genome Center, Ramathibodi Hospital reveals that scientists found the use of molnupiravir. Make COVID-19 mutate up to 100 times compared to the countries that use it. with not to use Based on more than 13 million samples from the global COVID database “GISAID” 2022 and research from The United Kingdom confirmed 4 cases in Thailand. How much will the epidemic spread? Severe or not ? with explanatory information
by Medical Genome Center Faculty of Medicine Ramathibodi Hospital Mahidol University posted information disclosure via the Center for Medical Genomics Facebook page on February 9, 2023 stating that
The Genome Center has begun tracking mutations in a specific pattern on the 2019-coronavirus genome due to use of antiviral drugs Molenupiravir (Molnupiravir) Research from the United Kingdom confirmed 4 cases in Thailand. (Update 9 Feb. 2023 time 8:17)
Molenupiravir (MoInupiravir) is an oral tablet. effective against virus originally developed for treatment influenza Later, it was found that the drug Molnupiravir can act against Several types of corona virus, such as SARS, MERS, and the virus. that causes COVID-19
If giving molnupiravir early of infection will reduce the severity of COVID-19 reduce the rate of serious illness Reduce the risk of hospital admission and reduce death by about 50 percent compared to those who did not use the drug.
Molenupiravir permission from FDA in the US, UK and Thailand in late 2021 and in Australia in early 2022.
Molenupiravir tablets It’s a synthetic imitation. Some structures of RNA which is genetic material in corona virus 2019 make viral genome mutation scattered until unable to divide infect (cells) in humans anymore
(Picture 1)

But some scientists worry that in some patients. Treatment with molnupiravir may not be able to eliminate the 2019 corona virus completely from the body Within 5 days, it could cause an epidemic. of corona virus 2019 mutated due to Molnupiravir can go up.
Dr Theo Sanderson of the Francis Crick Institute London and a research team from the UK. posted research results on the server of “medRxiv” in January 2023 (Not yet verified from the experts To be published in an academic journal) shows that from “scanning” or screening the genetic code The whole genome of COVID-19, more than 13 million samples from the global COVID-19 database “GISAID” in 2022, which is the time with the use of molnupiravir in many countries including Thailand
Found the genetic code of COVID-19 from many countries have a mutation in a specific manner indicating that an infected person has eaten Molenupiravir by found an epidemic It was a small cluster. ) was higher compared to unused infected molnupiravir in the treatment of
(Picture 2)

mutation Specifically (with higher G-to-A and C-to-T rates) found in countries where use is permitted. Molenupiravir such as the United States, the United Kingdom, Australia and Thailand are higher than other countries. not approved for use molnupiravir, such as France and Canada, up to 100 times
(Picture 3)

“According to the tracking date and location of the mutation on the genome line, it was found that Species with specific mutations due to Molenupiravir can be spread in the community, but not widespread.”
(Picture 4)
country example with approval for use Molenupiravir
+ Australia A specific mutation was found (presumed to be caused by the use of molnupiravir) 97 cases from the sample (uploaded) on the global COVID database “GISAID”), totaling 119,194 cases
+ USA 60 specific mutations were found from a total of 1,911,997 samples.
+ UK 23 specific mutations were identified from a total of 1,218,724 samples.
+ Japan 20 specific mutations were identified from a total of 321,520 samples.
+ Germany 10 specific mutations were identified from a total of 503,014 samples.
+ Israel Nine specific mutations were found out of a total of 107,477 samples.
+ Thai Four specific mutations were found out of a total of 21,459 samples.
+ etc.
Countries that are not allowed to use Molenupiravir
+ Canada One specific mutation was found out of the total 206,718 samples.
+ Finland Found 0 specific mutations out of 17,978 samples.
+ France Found 0 specific mutations out of 313,680 samples.
+ etc.
(Picture 5)

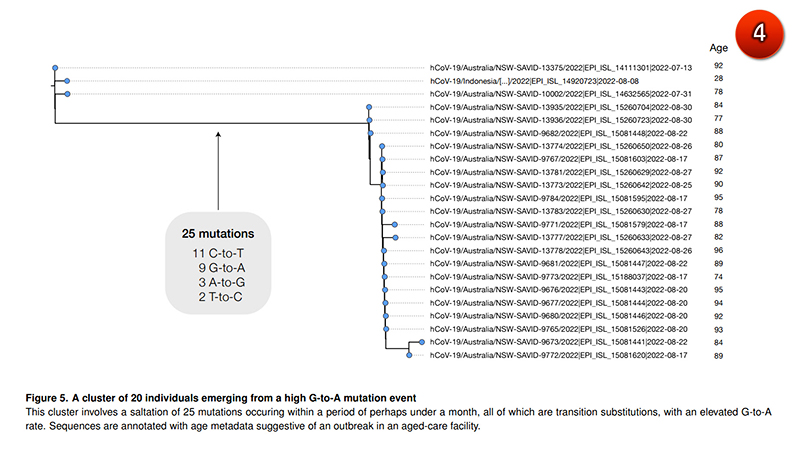
Specific mutations of COVID are also found in the elderly, who are more likely to eat. Molenupiravir more than young people and middle-aged people and in Australia which contains molnupiravir used in nursing homes 25 specific mutations of the virus were found. At least 20 people were found to spread, most of them the elderly in their 80s and 90s.
(Picture 4)

In conclusion, treatment with molnupiravir causing the virus to mutate greatly In most cases, the genome is damaged. can’t spread Inherit to the next generation But there is still a small part that even the genome has a lot of mutations. But still able to spread the epidemic in a limited area, which must continue to follow whether COVID-19 mutates from Molenupiravir to evade immunity Or is there a serious infection or is it just a weak virus? (From many mutations) and eventually disappeared.
Caution
The researcher uses the assumption that these mutations related to treatment with molnupiravir, but part of the information from the global COVID database “GISAID” does not have information that COVID-19 Treated with antiviral drugs? and what kind
Omicron since October 2022 there is a natural mutation Born into many subspecies (omicron soup) in the infected not receiving antiretroviral drugs as well
refer
Identification of a molnupiravir-associated mutational signature in SARS-CoV-2 sequencing databases
https://www.medrxiv.org/content/10.1101/2023.01.26.23284998v1
Could a popular COVID-19 antiviral supercharge the pandemic? From the journal Science
https://www.science.org/content/article/could-popular-covid-19-antiviral-supercharge-pandemic
Molnupiravir – an oral antiviral treatment for COVID-19
https://www.stellapharm.com/molnupiravir-an-oral-antiviral-treatment-for-covid-19
However, Backbone MCOT has information from the Department of Medical Services website that discloses information about clinical practice guidelines Diagnosis, treatment and prevention of hospital-acquired infections In case of Coronavirus Disease 2019 (COVID-19), revised dated 30 November 2022, as a recommendation the use of antiviral drugs in this group The antiretroviral drug Molnupiravir should be initiated within 5 days of onset of symptoms, 5 days of medication and 10 doses.
Read (full version) Information from the Department of Medical Services website.
+ Guidelines for clinical practice, diagnosis, treatment and prevention of hospital infections Coronavirus Disease 2019 (COVID-19) case for doctors and public health personnel, 26th edition, dated 30 November 2022
https://covid19.dms.go.th/Content/Select_Landding_page?contentId=180
#BackboneMCOT
References and thanks to information from:
Facebook: Center for Medical Genomics
(Medical Genome Center Faculty of Medicine Ramathibodi Hospital Mahidol University)
https://www.facebook.com/CMGrama
Website : Department of Medical Services
https://www.dms.go.th
