Updated:
Keep
A new innovative technique developed by a scientific team at the Center for Genomic Regulation (CRG) in Barcelona has discovered the existence of a multitude of ‘remote controls’ that control the function of proteins and that could be used as targets to achieve most effective and efficient drugs in pathologies as diverse as dementia, cancer and infectious diseases.
These ‘remote controls’ are scientifically known as allosteric sites. These are remote controls that “are distant from the site of action of the protein, but have regulatory or modulatory capacity of it”, explains to ABC Júlia Domingo, first co-author of the study, which is published this Wednesday in the journal “Nature”. And she adds a simile: “It’s as if with that remote control you could turn the light bulb on and off or regulate the intensity of the light.”
In this case, what is intended to be blocked or regulated is the activity of proteins that have their function altered in diseases. For example, in the case of cancer, the proteins that acquire mutations see their functionality altered, they do so abnormally and the cell grows in an unusual way. In many cases, there are no drugs that can modulate or block this abnormal activity or, if there are, they are not specific and also affect other proteins that function normally.
Traditionally, “drug hunters” have designed treatments that target a protein’s active site, the small region where chemical reactions take place or where targets bind. The drawback of these drugs, known as orthosteric drugsis that the active sites of many proteins are very similar and drugs tend to bind and inhibit many different proteins at the same time, even those that function normally and are not interesting to touch, which can cause side effects.
“This is where the allosteric concept comes in and the potential it has to design drugs. The interesting thing about allosteric sites is that they are super specific for each protein. If these allosteric sites find part of the protein surface where the drug can land, it will be extremely specific for that protein. We will be able to aspire to more effective medicines”, says the researcher.
“Not only do these potential therapeutic sites turn out to be abundant, but there is evidence that they can be manipulated in many different ways. Instead of just turning them on and off, we could modulate their activity like a thermostat. From an engineering point of view, it’s like we struck gold, because it gives us so much room to design ‘smart drugs’ that address the bad and omit the good”, explains André Faure, postdoctoral researcher at the CRG and first co-author of the article.
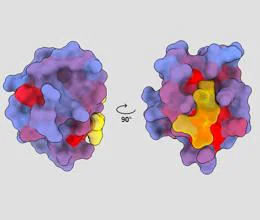
To reach this discovery, the team has used a method that has allowed them to take a protein and systematically and globally find all the allosteric sites. To do this, they have chosen two very abundant proteins in our human proteome. “50% of the protein surface has allosteric potential. Our method allows to atlas of allosteric siteswhich would make the process of searching for effective drugs much more efficient”, assures Júlia Domingo.
The study authors developed a technique called double-depth PCA (ddPCA), which they describe as a “brute force experiment.” “We break things on purpose in thousands of different ways to form a complete picture of how something works,” explains ICREA Research Professor Ben Lehner, Coordinator of the Systems Biology program at the CRG and author of the study. “It’s like if you suspect a spark plug is bad, but instead of checking just that, the mechanic would take the whole car apart and check all the parts one by one. By analyzing ten thousand things at once, we identify all the pieces that are really important.”
Then they used algorithms artificial intelligence to interpret laboratory results.
One of the great advantages of the method, in addition to simplifying the process necessary to find allosteric sites, is that it is a affordable and accessible technique for any research laboratory in the world. “They just need access to basic molecular biology reagents, access to a DNA sequencer and a computer. With these three components, any laboratory in 2-3 months, with a small budget, can carry out this experiment on the protein of interest that they want”, assures Júlia Domingo. The researchers’ hope is that other scientists will use the technique to quickly and comprehensively map the allosteric sites of human proteins one by one. “If we have enough data maybe one day we can go one step further and predict from the sequence of proteins to their function. Use this data to direct how we make better therapies or predict whether a certain change in a protein will degenerate into a disease”, concludes the researcher.
See them
comments
